Enantion Selective Synthesis of Chairal Alfa Amino Acids by Phase Transfer Catalysts
Haniyeh Alavi1, Milad Taheri* and Mehdi Daryani
Department of Chemistry, Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
Article Received on :
Article Accepted on :
Article Published : 19 Oct 2016
Amino Acids are consider as main base units forming peptide & Protein’s composites. They‘re playing important role in defining and forming or making them (Amino Acids) artificially in chemical labs. In this research, converting Glycine Amino Acid (without Chiral Carbon) to desirable Amino acids through proper C- alkylation’s process in salty chemical environment with present of phase transfer catalyst. With the present of Glycine as well as alkyl agent in Organic phase & base in water Phase and a Phase transfer catalyst forming Mono anion at phase as a mono alkylation Glycine product controlled production. As mentioned the aim of this research is the chiral amino acids synthesis. Glycine Amino acid is Chosen as a base abundant material in nature. Then every artificial compositions purified. Following compositions has recrystallization with petroleum ether and formed the production through chromatogram of (TLC), then detecting by comparing their RF with other composition’s RF to prove & support the production’s purifications grade. And then for final result, in order to define clearly and completely, 1H-NMR and other methods such as IR and Mass spectrums measuring we have been used. Achieved results from composed productions shows, their formations and performance is depended on proper composition periods (speed). s ed om composed es has been used
KEYWORDS:Chiral; Synthesis; α-Amino Acid; mono alkylation Glycine
Download this article as:| Copy the following to cite this article: Alavi H, Taheri M, Daryani M. Enantio Selective Synthesis of Chairal Alfa Amino Acids by Phase Transfer Catalysts. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Alavi H, Taheri M, Daryani M. Enantio Selective Synthesis of Chairal Alfa Amino Acids by Phase Transfer Catalysts. Available from: http://www.orientjchem.org/?p=22582 |
Introduction
Amino Acids are as base the units of lipids & proteins formations which they are used to make medicine. Relocating Amino acids molecules on chemical’s protein chains will produces new productions with new properties. Also, due to cell’s receptor in form of chiral, each Amino Acids enantiomers lead to new medicine or cause their effects. Therefore, producing new different Amino Acids enantiomers will design for new medicine. So, according to above information we have concluded the importance of the Amino Acids generation in chemical labs as for the medicine uses.
There are different types of Amino Acids. Except Amino Acid Glycine، Each Amino Acid made of an asymmetric Carbon called α- carbon which is banded in four different groups as Carboxyl (COOH) -Base Amine (NH2), a R- side chain and a Hydrogen band . most Amino Acids are participating in Synthesis Protein’s products. While the β-Amino Acids & γ and δ are intermediate chemical interaction. Most Amino Acids in PH level 7 are in shape of two poles means NH2⁺ and the other group COOH loosing the Hydrogen and show as COO⁻. Now if the NH2 agent respect to Alpha Amino Acid chain (in space figure) connect to Left side, it marked as (L) Amino Acid otherwise if the NH2 connect to right side of chain marked as (R).
Alpha Amino Acids is one of the important compounds in medicine productions industry. due to their strong and stable chemical transactions , and yet, easy biological accessibility, their synthesis production are the industries attention. Recently Scott & Ednel reported; they have synthesis Alpha Amino Acid from Acids and lipids solid phase state. they used “De Phenyl Imine Glycine Esther to synthesis the Alpha Amino Acid. As it known, they are different Amino Acids for different lipids, peptides and proteins base unites. So, as these fact each Amino Acids could leads to new/different medicine productions. It is another reasons synthesis Amino Acids is getting highly industries attention. It should pay attention, in plan to design and produce medicines, our body’s cell’s respirators are Chiral. Therefore used Amino Acids arrangement could produce different types of medicines with different effects. The goal of this research is also to covert the Amino Acids Glycine (Carbonless Chiral) to desirable Amino Acids with C- alkylation reaction in proper salty chemical environmental in present of glycine as catalyst. Also the Alkyl agent & a Catalyst in organic Phase and forming mono Anion it’s the mono alkylation Glycine synthesis result. This research’s renovation is controlling the amount of Base which react with Amino Acid and lead to forming Mono Anion and the mono mono alkylation Glycine chiral result. Notice; by using an organic Base LDA (Lithium Di Isopropyl) presenting in organic Phase at the same time, forming Di Alkyl Glycine is possible.
Experimental details
General method
All of reagents used in this research work were GR grade and all of solvents were distilled before use. Japanese weighing with 0.100 g sensivity model ANDGF-300, hitter and stirrer model Heidolphm Rn3004 Safty, Merk thin layer chromatography sheets Art no: 1:0554, Electrothermal melting point measurement apparatus , Memmert (oven) materials and glossy instruments desicator (oven), The structure elucidation was determined utilizing JEOL DELTA NMR 400 MHz (1H-NMR) and 100 MHz (13C-NMR) using CDCl3 and DMSO as solvent, and IR spectra were recorded on Perkin-Elmer-1420 Spectrophotometer. Spectrophotometer with using CDCl3 and DMSO as solvent, at University Shahr rey branch, and University of Tehran Iran.
Synthesis Methods
Methyl glycine family separately were synthesized and consequently, many new kind of imino ester glycine were obtained from these compounds.
General procedure for the Preparation of imino ester glycine
4 g (0/031mol) methyl glycine hydrochloride as the raw material used and the 3/370 g (0/031mol) benzaldehyde and 10 ml tree ethyl amine added and then 60 ml CH2Cl2 of the solvent used in this series 3 g Na2So4 Moreover, as a sorbent Add all the ingredients mixed together and 1 hour to make ends followed by Buchner magnesium sulfate eliminates hopper remove the funnel solution and by rotary solvent methylene chloride to remove then the organic phase Na2SO4 for dewatering Add funnel and chemical imino ester glycine was obtained.
To give (0.32 g, 90%); A white crystal; Mp: 205; IR (KBr): 3028, 2862, 1747, 1646, 644; 1 H-NMR (400 MHz, CDCl3) ä 3.65 (s, 3H), 4.51 (d, 2H), 7.29 (d, 3H), 7.62 – 7.62 (J =2 and 8 Hz, t, 2H), 8.12 (s, 1H); 13 C NMR (CDCL3) δ 51.6, 58.5, 128.8, 129.6, 139.7, 161.0, 170.3; MS m/z 177 (M+, 85.01); Anal.Calcd for C10H11NO2: C, 67.78; H, 6.26; N, 7.90; O, 18.60. Found: C, 67.98; H, 6.10; N, 7.85; O, 18.75.
Synthesis of imino ester glycine derivatives structure
Compound 1: To give (0.35 g, 95%); A white crystal; Mp: 108; IR (KBr): 3020, 2875, 1745, 1652, 710; 1 H-NMR (400 MHz, CDCl3) ä 1.46 (d, 3H), 3.67 (s, 3H), 4.14 (m, 1H), 7.29 (J =2 and 8 Hz, d, 3H), 7.62 – 7.64 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 18.0, 51.9, 66.6, 128.9, 129.2, 131.1, 139.9, 160.9, 171.4; MS m/z 191 (M+, 92.16); Anal.Calcd for C11H13NO2: C, 69.09; H, 6.85; N, 7.32; O, 16.73. Found: C, 67.98; H, 6.69; N, 7.08; O, 16.20.
Compound 2: To give (0.32 g, 85%); A White crystal; Mp: 181; IR (KBr): 3125, 2935, 1745, 1595, 659; 1 H-NMR (400 MHz, CDCl3) ä 2.7 (t, 3H), 2.8 – 3.1 (m, 2H), 3.67 (s, 3H), 7.08 (m, 1H), 7.12 (m, 2H), 7.21- 7.24 (m, 2H), 7.30- 7.32 (J =2 and 8 Hz, q, 5H), 7.50 (d, 1H); 13 C NMR (CDCL3) δ 34.00, 42.3, 51.9, 122.3, 127.3, 127.8, 128.7, 130.1, 139.5, 149.2, 163.4, 170.01; MS m/z 267 (M+, 90.01); Anal.Calcd for C17H17NO2: C, 76.38; H, 6.41; N, 5.24; O, 11.97. Found: C, 75.98; H, 6.52; N, 5.32; O, 12.10.
Compound 3: To give (0.40 g, 99%); A brown crystal; Mp: 217; IR (KBr): 3025, 2920, 1732, 1610, 700; 1 H-NMR (400 MHz, CDCl3) ä 2.55- 2.80 (m, 2H), 3.67 (s, 3H), 4.01- 4.06 (d, 2H), 4.97- 5.03(m, 1H), 5.70 (q, 1H), 7.29 (d, 3H), 7.62 (t, 2H), 8.09- 8.11 (J =2 and 8 Hz, s, 1H); 13 C NMR (CDCL3) δ 38.9, 51.9, 70.6, 116.4, 128.9, 129.2, 131.1, 134.4, 139.9, 1160.9, 171.4; MS m/z 217 (M+, 85.20); Anal.Calcd for C13H15NO2: C, 71.87; H, 6.96; N, 6.45; O, 14.73. Found: C, 71.98; H, 6.54; N, 6.50; O, 14.20.
Compound 4: To give (0.30 g, 80.3%); A Yellow crystal; Mp: 191; IR (KBr): 3028, 2835, 1740, 1635, 710; 1 H-NMR (400 MHz, CDCl3) ä 0.96 (t, 3H), 2.04 (m, 2H), 3.67 (s, 3H), 3.96 (t, 1H), 7.29- 7.31 (J =2 and 8 Hz, d, 3H), 7.62 (t, 2H), 8.13 (s, 1H); 13 C NMR (CDCL3) δ 18.9, 23.9, 51.9, 71.7, 128.9, 129.2, 131.1, 139.9, 160.9, 171.4; MS m/z 205 (M+, 78.96); Anal.Calcd for C10H21NO2: C, 70.22; H, 7.37; N, 6.82; O, 15.59. Found: C, 70.10; H, 7.89; N, 6.24; O, 15.10.
Compound 5: To give (0.38 g, 98%); A yellow crystal; Mp: 244; IR (KBr): 3025, 2870, 1735, 1642, 725; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 5.23- 5.25 (J =2 and 8 Hz, d, 1H), 7.28- 7.29 (d, 3H), 7.62- 7.64 (t, 2H), 8.11 (s, 1H), 8.60 (d, 2H); 13 C NMR (CDCL3) δ 51.9, 68.5, 124.3, 128.9, 129.2, 131.1, 139.9, 146.5, 149.9, 160.9, 171; MS m/z 254 (M+, 99.05); Anal.Calcd for C15H14N2O2: C, 70.85; H, 5.55; N, 11.02; O, 12.58. Found: C, 70.54; H, 5.98; N, 10.97; O, 12.10.
Compound 6: To give (0.31 g, 89%); A yellow crystal; Mp: 250; IR (KBr): 3125, 2970, 1725, 1632, 715; 1 H-NMR (400 MHz, CDCl3) ä 2.35 (s, 6H), 3.67 (s, 3H), 4.70- 4.73 (t, 2H), 6.88 (m, 1H), 6.92- 6.93 (t, 1H), 7.00- 7.01 (J =2 and 8 Hz, s, 2H), 7.09 (t, 1H), 7.29 (d, 3H), 7.62 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 24.6, 40.7, 51.9, 71.9, 126.6, 128.9, 129.3, 130.1, 135.9, 138.9, 139.9, 140.02, 142.9, 160.9, 171.4; MS m/z 371 (M+, 57.98); Anal.Calcd for C25H25NO2: C, 80.83; H, 6.78; N, 3.77; O, 8.61. Found: C, 80.56; H, 6.82; N, 3.46; O, 8.56.
Compound 7: To give (0.34 g, 93%); A yellow crystal; Mp: 238; IR (KBr): 3120, 2872, 1720, 1630, 714; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 3.73 (d, 6H), 4.00 (2H), 4.70- 4.73 (t, 2H), 6.59 (m, 1H), 6.63 (m, 1H), 6.68 (J =2 and 8 Hz, t, 1H), 6.72 (m, 2H), 7.01 (s, 2H), 7.10 (d, 1H), 7.29 (t, 3H), 7.62 (J =2 and 8 Hz, t, 2H) , 8.11 (s, 1H); 13 C NMR (CDCL3) δ 40.7, 51.9, 55.9, 71.9, 111.8, 112.3, 114.8, 120.6, 128.9, 129.3, 130.3, 131.2, 135.3, 139.9, 144.2, 158.2, 160.9, 161.2, 171.4; MS m/z 403 (M+, 90.16); Anal.Calcd for C25H25NO4: C, 74.42; H, 6.25; N, 3.47; O, 15.86. Found: C, 74.20; H, 6.30; N, 3.52; O, 15.59.
Compound 8: To give (0.29 g, 81%); A white crystal; Mp: 234; IR (KBr): 3122, 2870, 1721, 1633, 715; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 4.70- 4.73 (J =2 and 8 Hz, t, 2H), 7.08 (m, 2H), 7.12 (m, 4H), 7.21- 7.23 (m, 4H), 7.29 (J =2 and 8 Hz, t, 3H), 7.62 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 40.4, 51.9, 71.9, 126.7, 128.9, 129.3, 131.1, 139.9, 160.9, 171.4; MS m/z 343 (M+, 85.12); Anal.Calcd for C23H21NO2: C, 80.44; H, 6.16; N, 4.08; O, 9.32. Found: C, 79.54; H, 6.12; N, 4.12; O, 9.40.
Compound 9: To give (0.30 g, 82%); A white crystal; Mp: 198; IR (KBr): 3125, 2875, 1723, 1637, 710; 1 H-NMR (400 MHz, CDCl3) ä 2.35 (s, 3H), 3.65 (s, 3H), 5.20- 5.25 (J =2 and 8 Hz, d, 1H), 6.94 (s, 4H), 7.29 (t, 3H), 7.62- 7.64 (J =2 and 8 Hz, t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 24.3, 51.9, 68.5, 128.9, 129.2, 129.7, 131.1, 135.5, 137.2, 139.9, 16.9, 173; MS m/z 267 (M+, 97.13); Anal.Calcd for C17H17NO2: C, 76.38; H, 6.41; N, 5.24; O, 11.97. Found: C, 76.20; H, 6.20; N, 5.20; O, 11.93.
Compound 10: To give (0.33 g, 90%); A White crystal; Mp: 229; IR (KBr): 3025, 2878, 1729, 1629, 678; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 5.03 (t, 1H), 5.25 (J =2 and 8 Hz, d, 1H), 6.61 (t, 2H), 6.89 (d, 2H), 7.30 (J =2 and 8 Hz, t, 3H), 7.62 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 51.9, 68.9, 116.3, 128.9, 129.2, 131.1, 139.9, 157.3, 160.9, 173.00; MS m/z 269 (M+, 85.45); Anal.Calcd for C16H15NO3: C, 71.36; H, 5.61; N, 5.20; O, 17.82. Found: C, 71.20; H, 5.85; N, 5.15; O, 17.80.
Compound 11: To give (0.31 g, 89%); A yellow crystal; Mp: 196; IR (KBr): 3028, 2970, 1730, 1632, 700; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 3.73 (d, 3H), 5.24- 5.25 (J =2 and 8 Hz, d, 1H), 6.65 (2H), 6.95 (s, 4H), 7.28 (t, 3H), 7.63 (t, 2H), 8.10- 8.12 (J =2 and 8 Hz, s, 1H);13 C NMR (CDCL3) δ 51.9, 55.9, 68.5, 114.7, 128.9, 129.3, 130.8, 131.5, 139.8, 160.9, 173; MS m/z 283 (M+, 90.10); Anal.Calcd for C17H17NO3: C, 72.07; H, 6.05; N, 4.95; O, 16.94. Found: C, 72.10; H, 6.01; N, 4.90; O, 17.01.
Compound 12: To give (0.34 g, 93%); A white crystal; Mp: 244; IR (KBr): 3032, 2969, 1745, 1660, 723; 1 H-NMR (400 MHz, CDCl3) ä 3.69 (s, 3H), 5.23- 5.24 (d, 1H), 6.60 J =2 and 8 Hz, (t, 1H), 6.72 (d, 1H), 6.91 (d, 1H), 7.29 (t, 3H), 7.62 (J =2 and 8 Hz, t, 1H), 8.12 (s, 1H); 13 C NMR (CDCL3) δ 51.8, 68.5, 123.3, 126.9, 128.9, 129.2, 131.5, 139.8, 161.0, 171.5; MS m/z 259 (M+, 58.52); Anal.Calcd for C14H13NO2S: C, 64.84; H, 5.05; N, 5.40; O, 12.58; S, 12.36. Found: C, 64.80; H, 5.12; N, 5.42; O, 12.34; S, 12.20.
Compound 13: To give (0.32 g, 89%); A yellow crystal; Mp: 186; IR (KBr): 3050, 2932, 1739, 1635, 723; 1 H-NMR (400 MHz, CDCl3) ä 3.68 (s, 3H), 5.25 (J =2 and 8 Hz, d, 1H), 7.06- 7.07 (m, 3H), 7.14 (m, 2H), 7.30 (t, 3H), 7.62- 7.64 (J =2 and 8 Hz, t, 2H), 8.13 (s, 1H); 13 C NMR (CDCL3) δ 51.7, 68.8, 127.3, 128.5, 129.4, 131.1, 138.4, 139.8, 161.8, 173.5; MS m/z 253 (M+, 85.13); Anal.Calcd for C16H15NO2: C, 75.87; H, 5.97; N, 5.53; O, 12.63. Found: C, 74.95; H, 5.98; N, 5.10; O, 12.10.
Compound 14: To give (0.27 g, 75%); A brown crystal; Mp: 192; IR (KBr): 3031, 2966, 1747, 1661, 725; 1 H-NMR (400 MHz, CDCl3) ä 3.68 (s, 3H), 4.01- 4.05 (J =2 and 8 Hz, t, 2H),5.25 (d, 1H), 6.34 (d, 2H), 6.81- 6.83 (J =2 and 8 Hz, d, 2H), 7.27 (t, 3H), 7.65 (t, 1H), 8.13 (s, 1H); 13 C NMR (CDCL3) δ 51.7, 68.5, 116.8, 128.9, 129.2, 130.6, 138.5, 147.5, 160.9, 171; MS m/z 268 (M+, 75.52); Anal.Calcd for C16H16N2O2: C, 71.62; H, 6.01; N, 10.44; O, 11.93. Found: C, 71.45; H, 6.10; N, 10.52; O, 11.85.
Compound 15: To give (0.33 g, 85%); A brown crystal; Mp: 204; 3028, 2970, 1730, 1632, 700; 1 H-NMR (400 MHz, CDCl3) ä 2.35 (s, 3H), 3.67 (s, 3H), 5.25- 5.27 (J =2 and 8 Hz, d, 1H), 7.12 (d, 4H), 7.29 (t, 3H), 7.36- 7.38 (m, 4H), 7.62 (t, 1H), 8.12 (s, 1H); 13 C NMR (CDCL3) δ 24.3, 51.9, 68.5, 127.8, 128.9, 129.2, 131.1, 135.3, 137.4, 139.5, 160.9, 173; MS m/z 343 (M+, 58.35); Anal.Calcd for C23H21NO2: C, 80.44; H, 6.16; N, 4.08; O, 9.32. Found: C, 80.40; H, 6.12; N, 4.01; O, 9.20.
Compound 16: To give (0.33 g, 92%); A white crystal; Mp: 208; IR (KBr): 3122, 2870, 1721, 1633, 715; 1 H-NMR (400 MHz, CDCl3) ä 3.64 (s, 3H), 4.00 (t, 2H), 5.05 (t, 1H), 5.25- 5.27(d, 1H), 6.17 (t, 1H), 6.28- 6.31 (J =2 and 8 Hz, t, 2H), 7.29 (t, 3H), 7.62 (t, 1H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 51.7, 59.7, 116.2, 118.1, 123.00, 128.5, 129.8, 131.1, 139.8, 148.9, 160.7, 173.02; MS m/z 284 (M+, 90.16); Anal.Calcd for C16H16N2O3: C, 67.59; H, 5.67; N, 9.85; O, 16.88. Found: C, 67.54; H, 5.70; N, 9.99; O, 16.75.
Compound 17: To give (0.35 g, 95%); A yellow crystal; Mp: 197; IR (KBr): 3125, 2970, 1725, 1632, 715; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 4.00 (t, 2H), 5.25 (d, 1H), 6.73- 6.79 (s, 1H), 7.07 (t, 1H), 7.27- 7.29 (J =2 and 8 Hz, t, 4H), 7.62 (t, 2H), 8.13 (s, 1H);
13 C NMR (CDCL3) δ 51.9, 58.9, 110.6, 120.1, 122.8, 129.2, 129.7, 131.1, 139.9, 148.5, 150.9, 161.7, 172.9; MS m/z 313 (M+, 99.10); Anal.Calcd for C16H15N3O4: C, 61.34; H, 4.83; N, 13.41; O, 20.43. Found: C, 61.30; H, 4.90; N, 13.46; O, 20.34.
Compound 18: To give (0.33 g, 90%); A brown crystal; Mp: 188; 3032, 2969, 1745, 1660, 723; 1 H-NMR (400 MHz, CDCl3) ä 3.65 (s, 3H), 5.24- 5.25 (d, 1H), 7.28- 7.29 (J =2 and 8 Hz, t, 3H), 7.32 (t, 2H), 7.62 (t, 2H), 8.07 (d, 2H), 8.12 (s, 1H); 13 C NMR (CDCL3) δ 51.9, 68.8, 121.3, 128.9, 129.8, 130.6, 139.9, 147.5, 160.9, 171; MS m/z 298 (M+, 92.18); Anal.Calcd for C16H14N2O4: C, 64.42; H, 4.73; N, 9.39; O, 21.50. Found: C, 64.45; H, 4.72; N, 9.40; O, 21.45.
Compound 19: To give (0.27 g, 75%); A white crystal; Mp: 207; 3125, 2875, 1723, 1637, 710; 1 H-NMR (400 MHz, CDCl3) ä 3.60 (s, 1H), 3.66 (s, 3H), 7.12 (t, 2H), 7.30 (m, 5H),7.36 (m, 2H), 7.50 (d, 1H), 8.00- 8.01 (J =2 and 8 Hz, d, 1H), 8.90 (s, 1H); 13 C NMR (CDCL3) δ 43.4, 51.9, 119.5, 122.3, 127.9, 130.2, 132.6, 139.5, 143.1, 149.0, 154.8, 163.8, 170.0; MS m/z 336 (M+, 95.45); Anal.Calcd for C19H16N2O2S: C, 67.84; H, 4.79; N, 8.33; O, 9.51; S, 9.53. Found: C, 67.80; H, 4.80; N, 8.35; O, 9.45; S, 9.20.
Compound 20: To give (0.38 g, 98%); A yellow crystal; Mp: 296; IR (KBr): 3050, 2932, 1739, 1635, 723; 1 H-NMR (400 MHz, CDCl3) ä 2.35 (s, 3H), 3.66 (s, 3H), 5.26 (J =2 and 8 Hz, d, 1H), 7.12 (t, 2H), 7.26 (m, 1H), 7.29 (t, 3H), 7.36 (m, 2H), 7.62 (J =2 and 8 Hz, t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 17.9, 51.9, 123.4, 127.3, 128.9, 129.2, 130.5, 131.1, 138.5, 139.9, 160.9, 165.00, 167.1, 173.01; MS m/z 350 (M+, 85.16); Anal.Calcd for C20H18N2O2S: C, 68.55; H, 5.18; N, 7.99; O, 9.13; S, 9.15. Found: C, 68.75; H, 5.30; N, 8.10; O, 9.20; S, 9.25.
Compound 21: To give (0.33 g, 85%); A yellow crystal; Mp: 202; IR (KBr): 3022, 2923, 1740, 1630, 742; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 3.75(s, 3H), 5.26 (d, 1H), 7.12 (t, 2H), 7.26 (m, 1H), 7.29 (J =2 and 8 Hz, t, 3H), 7.36 (m, 2H), 7.62 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 51.9, 55.6, 68.5, 123.5, 127.8, 128.6, 129.5, 130.3, 131.1, 132.6, 138.5, 139.8, 157.0, 160.8, 173.1; MS m/z 366 (M+, 93.10); Anal.Calcd for C20H18N2O3S: C, 65.55; H, 4.95; N, 7.64; O, 13.10; S, 8.75. Found: C, 64.50; H, 4.98; N, 7.69; O, 12.98; S, 8.70.
Compound 22: To give (0.38 g, 98%); A white crystal; Mp: 204; 3031, 2966, 1747, 1661, 725; 1 H-NMR (400 MHz, CDCl3) ä 3.67 (s, 3H), 5.00 (t, 1H), 5.26 (J =2 and 8 Hz, d, 1H), 7.13 (t, 2H), 7.2 (m, 1H), 7.29 (t, 3H), 7.36 (J =2 and 8 Hz, m, 2H), 7.62 (t, 2H), 8.11 (s, 1H); 13 C NMR (CDCL3) δ 51.6, 68.5, 123.4, 127.9, 128.8, 129.2, 130.7, 131.4, 132.4, 138.1, 139.9, 157.1, 160.9, 173.4; MS m/z 352 (M+, 95.05); Anal.Calcd for C19H16N2O3S: C, 64.76; H, 4.58; N, 7.95; O, 13.62; S, 9.10. Found: C, 64.70; H, 4.98; N, 7.69; O, 13.58; S, 9.05.
Result and discussion
Chiral alpha amino acids are widely used in making medicine and poly proteins & poly peptides and yet much more in progress of producing on the way. Late result shows is not easy controllable applying Organic Base in order to preparing Mono Anion to achieve Mono alkylation instead the result would be a mixture of mono and di Alkylation products. While to gain the Mono Alkylation products, it needs matching mixing Base & Imine Glycine gradually applying double immiscible solution in which it has Base in water phase and Imine in Organic Phase and the process will gradually transmitting the Base from water phase to Organic Phase in it respond limited forming Anion and formed finally Mono glycine. through these process with Imine synthesis resulted from Benzaldehyde & Methyl ester glycine with present of methyl chloride solution. In this process Color Hydrate glycine salt has been used. The used Base to free the Amine Agent is Three Ethyl Amine. Remember this transaction with presence of different chemical solution, Salts Bases and different temperatures conditions have been researched and study according available documents. Following Amine Synthesis transaction, C- Alkylation from Amino Ester, Glycine Methyl Ester and Benzaldehyde made at Ednel Conditions. Performed Transaction with ethyl iodide shows probable combination, but with high degree impurity of both products is possible. It is suggested to continue these process could completed and saved possibly use the phase transfer catalyst with proper design and Base’s agents which has high different Basic power and also Organic Solutions Phase followed by heat and saved Alkylation .
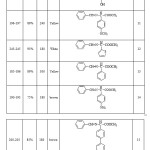 |
Table 1 Click here to View table |
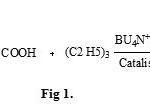 |
Figure 1: Click here to View figure |
 
 |
Figure 2 Click here to View figure |
Conclusions
The purpose of this project is to prepare the synthetic materials in which they are used in medicine. In This method of preparing Imine could proceed at common room temperature & no need special processing environments conditions. using different types of Alkyl halide to gain different products used in new medicine. And using different type of solutions could effect to improve the production’s process performance quality. Thus using the different types of phase transfer catalyst for different performances are applied besides of simplicity, Applying These methods also speed up the processing mechanism. as the production result proves, high speed composed materials production’s purity formed better and sorting them are much easier.
Acknowledgements
We thanks to Mr. Hanafi for his technical supports in IR Spectroscopy of synthesized products at Islamic Azad University, Shahr rey branch.
References
- M.J. Zhou, C. Scott, W.L.J. Am, Chem. Soc, 118: 6070-6071 (1996).
- Z. Andrade, Z. Suarez, Synthesis. Lett, 12: 2135-2138 (2004).
- R. S. Vaas, J. Dudas, R. S. Varma, Tetrahedron. Lett, 40: 4951-4954 (1999).
- R. S. Varma, R. Dahiya, S. Kumar, Tetrahedron. Lett, 38: 2039-2042 (1997).
- M. J. O’Donnell, S. Wu, J. C. Huffman, Tetrahedron. Lett, 50: 4507-4518 (1994).
- M. J. O’Donnell, W. D. Bennett, S. Wu, J. Am. Chem. Soc, 111: 2353-2355 (1989).
- G. Stork, A. Y. W. Leong, A. M. Touzin, J. Org. Chem, 41: 3491–3493 (1976).
- M. J. O’Donnell, J. M. Boniece, S. E. Earp, Tetrahedron. Lett, 19: 2641–2644 (1978).
- M. J. O’Donnell, T. M. Eckrich, Tetrahedron. Lett, 19: 4625-4528 (1978).
- (a): M. J. O’Donnell, K. Wojciechowski, Synthesis. Lett, 6: 313-315 (1984).
- W. Scott, M. J. Zhou, C. Fang, M. J. O’Donnell, Tetrahedron. Lett, 38: 3695-3698 (1997).
- M. J. O’Donnell, F. Delgado, R. F. Pottorf, Tetrahedron. Lett, 55: 6347-6362 (1999).
- Lygo. Barry, I. Benjamin,; Acc. Andrews, Chemical . Research, 37 (8): 518–525 (2004).
- J. Martin,; M. J. O’Donnell, Chemical Research, 37 (8): 506–517 (2004).
- M. Keiji, O. Takashi, Chemical. Reviews, 103 (8): 3013–30 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









