Electrochemical Behavior of Silver in Sodium Sulphate Solution during Anodic Polarization Revisited
Bayeshov Abduali1 , Tuleshova Elmira1 and Tukibayeva Ainur2*
1Department of Ecology and Chemistry, A.Yassavi International Kazakh-Turkish University, 2Department of Nanotechnology, M.Auezov South Kazakhstan State University, 5 Tauke-khan ave., Shymkent, 160000, Kazakhstan.
The results of studying the electrochemical behavior of silver in sodium sulphate solution during anodic polarization are given in this work. The investigation on studying electrochemical properties of silver has been carried out by the use of cyclic voltammetric method. It was investigated that complicated electrochemical reactions occur in sodium sulphate solution using silver electrodes, in anodic area takes place the silver oxidation with formation of oxides and sulphate of silver, and in cathodic area the oxygen compounds and silver sulphate regenerate.
KEYWORDS:Anode polarization; Electrochemical behavior; Potentiodynamic polarization curves; Silver
Download this article as:| Copy the following to cite this article: Abduali B, Elmira T, Ainur T. Electrochemical Behavior of Silver in Sodium Sulphate Solution during Anodic Polarization Revisited. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Abduali B, Elmira T, Ainur T. Electrochemical Behavior of Silver in Sodium Sulphate Solution during Anodic Polarization Revisited. Available from: http://www.orientjchem.org/?p=11938 |
Introduction
The process of formation of oxides on metals and alloys constantly causes the interest of researchers. Oxide accumulation on an electrode surface in anodic dissolution or corrosion usually results in revolutionary changes in kinetics of these processes, and often determines the possibility of their practical use as in case of Al, Ti, Mg and other metals 1-6. Formation of oxides, and in some cases, dissolution of oxides in order to obtain pure metals is of special interest. We previously conducted a number of researches on an electrochemical behavior of various metals, particularly silver, in the alternating current polarization of the commercial frequency7-10. The peculiarity of the processes proceeding under the commercial alternating current is that commutation produces interesting results. For instance, in case of titanium and aluminum it is possible to skim an oxide film and create conditions for a further smooth dissolution of metals with subsequent synthesis of their soluble salts. Such intensive dissolution of “passive” metals can be easily explained by the fact that the oxide film formed in the anodic half-cycle of the alternating current undergoes recovery in the cathodic half-cycle, and after several such rotations a surface of a metal is completely cleaned from the oxide film, and metal ions transit into a solution. These processes occur very intensively, as commutation occurs rapidly (50 times per second). Thus, conducting research on kinetics of the process during its flow is extremely complicated. Thereupon, we have attempted to explain occurring processes using a classical electrochemical method – a cyclic voltammetric method.
The scientists11–16 investigated the anodic dissolution of silver under steady-state polarization in neutral and acidic solutions revealed that the process rate is limited by diffusion removal of simple or complex silver ions in the solution. Sufficiently positive potentials, passivating films form at silver surface. These have an increased number of macrodefects (porosity)13.
The authors16,17 showed that in neutral and weakly acidic sulphate media, the silver passivation is attributed to the formation of a silver sulphate layer.
The aim of the paper is to study the influence of the concentrations of sodium sulphate and potential sweep rates on the anodic oxidation of silver under potentiodynamic polarization.
Material and Methods
We have detected anode-cathode and cathode-anode cyclic potentiodynamic polarization curves with a potentiostat SVA-1BM. A flat end of a silver core with a superficial dimension of 4 square millimeters is used as a test electrode. A silver chloride electrode is used as a reference electrode (all the main electrode potential values are given in relation to this electrode), and platinum is taken as an auxiliary electrode.
Results and Discussion
Typical cyclic voltammetric curves of oxidation-reduction process of silver in 0.5M sodium sulphate solution at the scan rate of 10 mVsec-1 are shown in Figure 1.
Anodic branch of the cyclic current-voltage curve is the peak current, reflecting the silver oxidation process according to the known reaction (Fig. 1, Curve 1, Peak ‘a’):
![]()
An offset of the potential to a more positive side leads to degradation of the current magnitude apparently connected with formation of a passive film of silver sulphate on the electrode surface.
In the potential range +1.6 … +1.8 V the curve is characterized by the peak reflecting oxygen precipitation. During these potentials the following reactions of oxides formation may occur on the electrode surface:
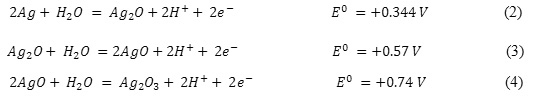
An electrode polarization in a reverse cathodic direction is described by the polarogram with one distinct anomalous anodic peak current (Figure 1, Curve 1, Peak ‘b’).
 |
Figure 1; The anode-cathode (1) and cathode-anode (2) cyclic potentiodynamic polarization curves of silver in 0.5molL-1 sodium sulphate solution. Click here to View figure |
Anomalous anodic peaks in cathodic potentiodynamic curves of metals were previously observed. For instance, in works by Brainina and others18 such phenomenon was observed in metals of the copper subgroups in cathodic potential sweep after preliminary anodic polarization. As they rightly pointed out, appearance of the anodic peak is related to formation of oxide and/or salt film and explained by a further oxidation of transient low-valence silver ions to Ag (II).
Also in work19 appearance of the anodic peak, abnormal for the cathodic potentiodynamic curve, was detected during the cathodic polarization of close-packed and powdered titanium electrodes in sulphuric acid solution.
It was found that first a traditional reduction current of a titanium oxide film is observed in a titanium cathodic polarogram, then anomalous current in the form of the anodic peak appears. The authors of the work believe that the detected anodic current is in essence a wave of transit of a titanium oxide electrode into a titanium hydride electrode. It was established that at a high potential sweep rate this anomalous wave in the polarogram “skips”, i.e. is not fixed. Substantially different nature of the anodic peaks, noted in works18,19, in this case may apparently have one common explanation. In every instance, anodic peaks are natural phenomena occurring in a case when an electrode or surface layers of an electrode are thermodynamically unstable in a certain area of potentials.
Further polarization in the cathodic direction results in appearance of reduction waves of silver-oxygen compounds by the reactions (5,6), reduction waves until metallic silver by the reaction (7) (Curve 1, Peaks ‘c’, ‘e’, ‘f’), and reduction waves of formed silver sulphate by the reaction (8) (Curve 1, Peak ‘d’). At a potential of -1.25 V hydrogen is disengaged. Conclusions about the possibility of the specified reaction behavior are made according to the analysis of the literature data20 and polarization curves.
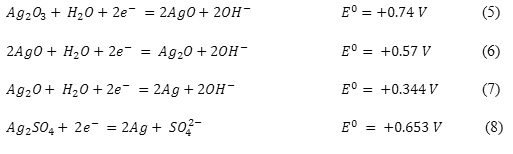
The results of the research on the influence of concentration of sodium sulphate on the metal dissolution rate are presented in Figure 2.
![Figure 2: The anodic polarization curve of silver in various concentrations of sodium sulphate (a) and the logarithmic dependence of the current peak height of oxidation of silver on the concentration of sodium sulphate (b): [Na2SO4], molL-1: 1 – 0.5; 2 – 1.0; 3- 1.5; 4 – 2.0; 5 – 2.5.](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Elec_Baye_fig2-150x150.jpg) |
Figure 2: The anodic polarization curve of silver in various concentrations of sodium sulphate (a) and the logarithmic dependence of the current peak height of oxidation of silver on the concentration of sodium sulphate (b): [Na2SO4], molL-1: 1 – 0.5; 2 – 1.0; 3- 1.5; 4 – 2.0; 5 – 2.5. Click here to View figure |
A cross-plot lgi-lg[Na2SO4] has a fracture indicative of the changes of electrode process mechanism in elevated concentration of sulphate – ions. The reaction order of silver oxidation is 0.27.
Anodic polarization curves of silver at various potential sweep rates ranged from 5 to 100 mVs were detected in 0.5molL-1 sodium sulphate solution (Figure 3).
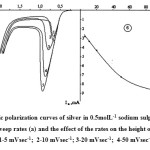 |
Figure 3: The anodic polarization curves of silver in 0.5molL-1 sodium sulphate solution at various potential sweep rates (a) and the effect of the rates on the height of the current peak oxidation (b): V: 1-5 mVsec-1; 2-10 mVsec-1; 3-20 mVsec-1; 4-50 mVsec-1; 5-100 mVsec-1. Click here to View figure |
With increasing potential sweep rate the current peak height increases. The dependence of the current peak on an electric potential sweep rate is of a nonlinear character (Figure 3 (b)). Electronic processes are possibly controlled by electron transfer kinetics and a diffusion rate simultaneously. The researches on identification of structure-sensitive characteristics of nanoscale anodic oxide Ag (I) were conducted. In particular, the authors21 established that the silver oxide anodically formed in an alkali solution (I) is structurally disordered, representing a N-type semiconductor with predominance of donor defects. In our researches the formation of oxides with disordered structure that affects a character of a current peak dependence on the sweep rate possibly also occurs.
Conclusion
Thus, with the method of detecting the cyclic potentiodynamic polarization curves it is shown that complicated electrochemical reactions occur in sodium sulphate solution using silver electrodes, in the anodic area silver oxidation with formation of oxides and sulphate of silver takes place, and in the cathodic area oxygen compounds and silver sulphate regenerate. These and other data received from the previous researches7-9,22 form the theoretical basis of the electrode processes, occurring in the alternating current polarization of silver and other noble metals, and serve as the prerequisite for developing new methods of metal extraction from barren liquors and drain water, methods of synthesis of a number of compounds that have widespread application in various sectors of industry.
References
- Zhuk N.P., Course of the theory of corrosion and protection of metals, Alliance, Мoscow, 472 (1976)
- Skorcheletty V.V., Theoretical foundations of corrosion of metals, Chemistry, Leningrad, 264 (1973)
- Keshe G., Corrosion of metals, Physicochemical principles and current problems, Metallurgy, Moscow, 214 (1984)
- Semenova I.V., Floriyanovich G.M., Khoroshilov A.V., Corrosion and corrosion prevention, Physmatlit, Moscow, 169 (2002)
- Basavanna S. and Arthoda Naik Y., Indian J Chem Technol, 19, 91(2012)
- Hackerman N., Corrosion Science, 7, 39(1967)
- Zhurinov M. Z., Baeshov A. B., Baeshova A. K., Iztleuov G. M., Reports of the Academy of Sciences of the Republic of Kazakhstan, 2, 5 (2006)
- Bayeshov A. B., Tuleshova E. Z., Bayeshova A. K., Zhandarbekova S. B., Reports of the Academy of Sciences of the Republic of Kazakhstan, 4, 103 (2005)
- Bayeshov A. B., Tuleshova E. Z., Bayeshova A. K., Chemical Journal of Kazakhstan, 4, 83(2006)
- Tuleshova E. Z., Bayeshov A. B., Bayeshova A. K., Actual problems of modern sciences, 1st International Forum, Samara, 2005
- Dmitrienko S. V., Molodov A. I., Losev V. V., Elektrokhimiya (Electrochemistry), 16, 895(1980)
- Anokhina I. V., Vvedensky A. V., Popova L. P., Marshakov I. K., Zashch. Met. (Protection of Metals), 5, 756 (1989)
- Vvedensky A. V., Stekol’nikov Yu. A., Tutukina N. M., Marshakov I. K., Elektrokhimiya (Electrochemistry), 18, 1646 (1982)
- Gordeeva T. V., Krasikov B. S., Zh. Prikl. Khim. (Journal of Applied Chemistry), 57, 2491(1984)
- Altukhov V. K., Shatalov V. G., Elektrokhimiya (Electrochemistry), 23, 968 (1987)
- Grishina E. P., Rumyantsev E. M., Russian Journal of Electrochemistry, 37, 409 (2001)
- Villulas H. M., Teijebo M., López, Abstracts of Papers, 43rd ISE Meet., Cordoba, 1992
- Brainina K. Z., Ashpuri V. V., Sokolov M. A., Electrochemistry, 17, 400(1981)
- Buketov E. A., Kozhakov B. E., Bayeshov A., Reports of the USSR Academy of Sciences, 265, 113(1982)
- Bahchisaraytsyan N.G., Borisoglebsky Y.V., Practicum in Applied Electrochemistry, Chemisty, Leningrad, 287 (1990)
- Kudriashev D. A., Grushevskaya S. N., Vvedensky A. V., Protection of metals, 43, 652(2007)
- Bayeshov A. B, Tuleshova E. Z, Bayeshova A. K, Kazakhstan N18396 RK, 16 April 2007

This work is licensed under a Creative Commons Attribution 4.0 International License.









