Effect of Drying on the Synthesis and Characterization of MoVTeNbOx Mixed Metal Oxide Catalysts Prepared by Reflux
Irmawati Ramli1,2*, Che Ku Nor Liana Che Ku Hitam1 , Hossein Abbastabar Ahangar1,3 and Abdul Halim Abdullah2
1 Centre of Excellence for Catalysis Science and Technology, 2Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. 3Materials Science and Engineering Department, Islamic Azad University, Najafabad Branch 85141-43131, Iran.
Article Received on :
Article Accepted on :
Article Published : 01 Mar 2013
MoVTeNb mixed oxide catalysts were successfully prepared by reflux with different drying methods. X-ray diffraction (XRD) confirmed the presence of orthorhombic (M1), hexagonal (M2) and (Mo0.93V0.07) 5 O14 phases after spray drying the slurry, while the catalysts obtained from the precipitate and mother liquid contained only hexagonal M2 and (Mo0.93V0.07) 5 O14 phases. Temperatureprogrammed reaction (TPRn) analysis and catalytic test identified the highest activity in the sample synthesized by spray drying in the partial oxidation of propane to acrylic acid.
KEYWORDS:MoVTeNb mixed metal oxide; reflux; propane oxidation
Download this article as:| Copy the following to cite this article: Ramli I, Hitam C. K. N. L. C. K, Ahangar H. A, Abdullah A. H. Effect of Drying on the Synthesis and Characterization of MoVTeNbOx Mixed Metal Oxide Catalysts Prepared by Reflux. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Ramli I, Hitam C. K. N. L. C. K, Ahangar H. A, Abdullah A. H. Effect of Drying on the Synthesis and Characterization of MoVTeNbOx Mixed Metal Oxide Catalysts Prepared by Reflux. Available from: http://www.orientjchem.org/?p=11929 |
Introduction
The partial oxidation of abundant and much cheaper alkanes to highly value oxygenates can bring huge economic incentives to industries and end users [1], one of which is the partial oxidation of propane to acrylic acid. The process has been the focus of extensive research since the early 1990s, due to its importance as a valuable intermediate for the preparation of fibres, synthetic rubber, and synthetic resins, among other things. [2,3]. The most effective catalysts to date in the propane selective oxidation to acrylic acid are MoVTeNbOx catalysts patented by Ushikubo et al. [4]. The high activity and selectivity of the catalyst are attributed to the presence of two crystalline phases, orthorhombic M1 and hexagonal M2. M1 is the most effective phase in the oxidation of propane to acrylic acid because it is the key paraffin activating catalyst. Its active centers contain all of the key elements, V5+, Te4+ and Mo6+. These active centers are properly arranged to catalytically transform propane into acrylic acid, and four Nb5+ centers surrounded by five molybdenum-oxygen octahedra. The M2 phase acts as co-catalyst which enhances the yield, due to its performance in converting the free propylene intermediate into acrylic acid [5].
In this study, a reflux method was used to synthesize the MoVTeNb oxides. This method is economical since the apparatus is simple and easy to assemble. It has the advantage of allowing intimate mixing of corresponding species in a homogeneous state at the solvent’s boiling point and under atmospheric pressure. It is also possible to combine the advantages of both hydrothermal and slurry methods via reflux [6]. Through reflux, the sample can be left for a long period of time without adding more solvent for fear that the reaction vessel will boil dry as any vapour immediately condenses. Additionally, the reaction proceeds at a constant temperature, as a given solvent will always boil at a certain temperature. The temperature can be controlled within a very narrow range by careful choice of the solvent. Although a magnetic stirring is often used to achieve a uniform solution, the constant boiling action also serves to continuously mix the solution. Under controlled conditions, this technique is useful for performing chemical reactions that require substantial time. Since reflux offers the possibility for observation and involvement, allowing interaction and more thorough inspection of the reaction medium, whose chemical and physical properties over the course of the reaction, so it could be a promising method to prepare this kind of catalyst [6].
Three different methods to remove water from the precursors have been used; spray drying, rotavap and oven drying. The physico-chemical properties and catalytic behavior of all catalysts were compared via XRD, BET, ICP-AES, H2-TPR and TPRn. Catalytic tests were performed to gain insight into the activity and selectivity of the synthesized catalysts for the conversion of propane to acrylic acid.
Experimental
Catalyst preparation
All reagents were commercially sourced and used without further purification. The MoVTeNbO catalyst was prepared by reflux (Fig. 1). Solid V2O5 followed by solid Te(OH)6 were added to an ammonium heptamolybdate tetrahydrate (AHM) solution at 80˚C. The MoVTe solution was cooled to room temperature. Aqueous ammonium niobium oxalate hydrate was added. The resulting orange slurry was placed in round bottom flask and refluxed at 80˚C for 48 h. The slurry was divided into two parts. One part was dried by spraying the slurry onto a hot plate at 180˚C (Spray-dried). The second part was centrifuged to separate the mixtures. The denser precipitate was dried in an oven (Oven-dried). The remaining solution was rotary evaporated, which produced a solid precipitate (Rotavap). All of these precursors were calcined in air at 280˚C for 1 h followed by calcinations in N2 at 600˚C for 2 h.
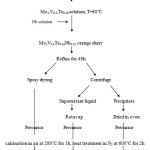 |
Figure 1: Flow chart of the synthesis of MoVTeNb mixed oxides. |
Characterization of samples
The phases of the catalysts were determined with an X-ray diffractometer (Cu Kα) by using a Shimadzu Model XRD 6000 Diffractometer. The specific surface area of the catalyst was measured by employing nitrogen adsorption using Sorptomatic 1990 series nitrogen adsorption/desorption analyzer. The composition of each element was determined by ICP-AES. The H2-TPR and TPRn experiments were performed using a ThermoFinnigan TPDRO 1110 Instrument. For H2-TPR, the pretreatment step was performed by heating about 0.01 g of the calcined sample from 50 to 120˚C in a flow of nitrogen (1 bar, 20 cm3 min-1) before cooling it to ambient temperature prior to TPR. The flow was switched to 5% hydrogen in argon (1 bar, 25 cm3min-1) and the temperature was increased from room temperature to 1000˚C at a rate of 10˚C min-1. A thermal conductivity detector (TCD) was used to measure the reduction in the sample by monitoring hydrogen consumption. For TPRn, the pretreatment step was performed by flowing 25 cm3 min-1 of argon over about 0.2 g of the calcined sample and held for 10 min. The sample was then purged with helium (25 cm3 min-1) and held for 30 min. After the pretreatment, the reaction was performed in a propane/helium mixed stream (5% propane, 1 bar, 10 cm3min-1)as the temperature ranged from 50 to 750˚C with a heating rate of 10˚C min-1. The Mass Spectrometer (Pfeiffer OmniStar), which is capable of monitoring multiple masses continuously, was used to detect the output gas.
Catalytic Tests
The catalytic experiments were performed in a fixed-bed Pyrex tubular reactor working at atmospheric pressure and a temperature range of 320 – 450˚C in order to achieve higher selectivity towards partial oxidation products. The heating rate of the furnace is 8 – 9˚C min-1 from ambient temperature to 380˚C and 4˚C min-1 from 380˚C to 420˚C. 0.5 g of catalyst was mixed with 3 g of quartz sand in a Pyrex tube and held by plugs of quartz wool with the total flow rate of 50 mL/min. The gas chromatography consisted of three types of columns in order to analyze the entire product. These three columns were Porapak QS, Molecular Sieve 13X, and Gaskuropak 54. Porapak QS is used to isolate the oxygenated products, which are acetone, acetic acid and acrylic acid while Molecular Sieve 13X and Gaskuropak 54 are used to separate gases such as O2, N2 and CO and differentiate between hydrocarbons and CO2. The catalytic reaction was started by introducing the feed consisting of propane, oxygen, nitrogen and steam (water) with flow rate of 50.0 mL min-1. In the feed composition of propane/oxygen/nitrogen/steam = 2/10/2.5/22.5, propane is flowed at the rate of 1.0 mL min-1, oxygen at 5.0 mL min-1, nitrogen at 1.3 mL min-1 and steam at 11.3 mL min-1.
Results and discussion
There were two broad bands centered at 2θ = 9.5-10.5 and 27-28˚ in the XRD patterns of MoVTeNbO precursors that are close to the reflections found for the crystalline Anderson-type compounds (NH4)6TeMo6O24.7H2O[7] and/or (NH4)7TeMo5VO24.8H2O[8] (Fig. 2).
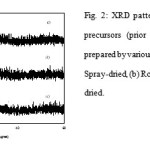 |
Figure 2: XRD patterns of MoVTeNbO precursors (prior to heat treatment) prepared by various drying methods: (a) Spray-dried, (b) Rotavap and (c) Oven-dried. |
XRD analysis indicates the heat treated catalysts are well-crystallized (Fig.3). The XRD patterns of the Spray-dried sampleshows the presence of the M1 phaseas evidenced by the patterns at 2θ = 6.6, 7.9, 9.0, 22.1, and 27.4˚[9]. In addition, peaks at 2θ = 22.1, 28.2, 36.2, 45.0, 50.0˚ and 2θ = 23.4, 25.1, 31.6˚ indicate the formation of M2 and (Mo0.93V0.07)5O14 phases respectively. In contrast, different XRD patterns were observed for RotavapandOven-dried samples where both indicate the presence of M2and (Mo0.93V0.07)5O14 [JCPDS-31-1437] phases [9].
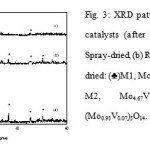 |
Figure 3: XRD patterns of MoVTeNbO catalysts (after heat treatment): (a) Spray-dried, (b) Rotavap and (c) Oven-dried: (♣)M1, Mo7.8V1.2NbTe0.94O29; (♦) M2, Mo4.67V1.33Te1.82O19.82; (♠) (Mo0.93V0.07)5O14. Click here to View figure |
Table 1 summarizes some of the main characteristics of MoVTeNb oxide catalysts. No significant differences in the BET surface area of the heat-treated samples. On the other hand, the composition of the Spray-dried sample is similar to the nominal composition of the raw materials (Mo1.0V0.30Te0.16Nb0.12) as analyzed via ICP-AES. A significant deviation from the intended nominal composition was observed for the Oven-dried and Rotavap samples. The Oven-dried sample showed an enrichment of Nb and a high depletion of Te. A different phenomenon was presented by the Rotavap sample where the depletion of Nb was observed while Te and V contents were quite similar to the nominal composition.
Table 1: Characteristic of the MoVTeNbO catalysts
|
Sample |
SBET (m2/g) |
Mo-V-Te-Nb atomic ratio a |
Crytalline phases b |
|
Spray dried |
7.0 |
Mo1.0V0.30Te0.16Nb0.12 |
Mo7.8V1.2NbTe0.94O29; Mo4.67V1.33Te1.82O19.82;(Mo0.93V0.07)5O14 |
|
Rotavap |
6.3 |
Mo1.0V0.33Te0.14Nb0.03 |
Mo4.67V1.33Te1.82O19.82;(Mo0.93V0.07)5O14 |
|
Oven dried |
6.7 |
Mo1.0V0.24Te0.04Nb0.28 |
Mo4.67V1.33Te1.82O19.82;(Mo0.93V0.07)5O14 |
a Chemical composition as determined by ICP-AES
b Observed by XRD.
The results indicate that spray-drying not only led to the inhibition of selective crystallization with uniform composition, but also to the similar distribution of metal ions in the Spray-dried sample and the starting gel [9]. Additionally, the compositional segregation in the precipitate occurred after centrifuge due to the formation of two or more metal oxide phases. In reflux, the change in the color of the slurry and composition of catalysts indicates chemical interactions between these metals differ after 48 h reflux. Also, the mother liquid did not show chemical complexity (XRD Fig.) that was previously reported by Lin in 2003[2].
Fig. 4 represents the hydrogen TPR profiles of Spray-dried, Rotavap and Oven-dried samples. Generally, all of the samples display two reduction peaks. The Spray-dried sample produced two overlapping peaks with a peak maximum at 606 and 695˚C, the Rotavap sample had peaks at 679 and 719˚C whereas the Oven-driedsample had two peaks at 500 and 613˚C. The reduction occurred in the temperature range of 500-720 ˚C. This could be related to the breakage of strongly bound oxygen species as indicated by the high reduction activation energy levels; 155 and 167 kJ/mol for Spray-dried, 167 and 172 kJ/mol for Rotavap,and 137 and 155 kJ/mol for Oven-dried samples might contribute to the selective oxidation products. It is a certainty that there are small reduction peaks in the reduction profiles of Spray-dried samples at temperatures below 400˚C. This could be due to the weakly adsorbed oxygen species that could contribute to the non-selective oxidation product.
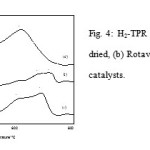 |
Figure 4: H2-TPR profiles for (a) Spray-dried, (b) Rotavap and (c) Oven-dried catalysts. |
Fig. 5 shows the temperature-programmed reaction (TPRn) profiles for the three catalysts. A mixture of 5% propane/He gases were passed through MoVTeNbO samples as the temperature increased from 50˚C to 750˚C at a heating rate of 10˚C min-1. The online mass spectrometer continuously recorded signals from propane (m/z=29), CO (m/z=28), propylene (m/z=41), CO2 (m/z=44), acrolein (m/z=56) and (acrylic acid (m/z=72).
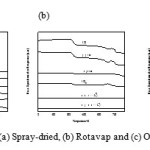 |
Figure 5: TPRn profile of (a) Spray-dried, (b) Rotavap and (c) Oven-dried catalysts. |
Desorption of propane, CO, CO2 and propylene was observed in the Spray-dried catalyst at a low temperature. This might have occurred when the propane reacted with the non-selective oxygen species with a small reduction profile at temperatures below 400˚C as shown by the H2-TPR results. The production of the acrolein intermediate and acrylic acid were observed at temperatures above 600˚C. The reduction profile of the H2-TPR analysis has two overlapping peaks, which indicates the presence of two kinetically different oxygen species in the Spray-dried catalyst. The catalyst is active and selective due to the M1 phase, which contains the desired metal ions that could contribute to the selectivity towards oxygen for the insertion.
Different phenomena occurred with the Rotavapand Oven-dried catalysts. At low temperatures, only the adsorption of propane occurred on the surface of the catalyst. The propane was not converted. As the temperature increased to 600˚C, all the physisorbed and chemisorbed propane desorbed from the catalyst and CO, propylene and CO2 were produced. Unfortunately the desired product, acrylic acid was not obtained. This proves that although the RotavapandOven-dried catalysts are active, they are non-selective towards the production of acrylic acid. These two samples also have two types of kinetically active, yet non-selective oxygen species in their reduction profiles.
The Spray-driedcatalyst has the highest activity of propane because it has the M1 phase, which is the key paraffin activating catalyst. Its active centers contain all of the key elements V5+, Te4+, Mo6+ that are properly arranged to catalytically transform propane into acrylic acid, and four Nb5+ centers, each surrounded by five molybdenum-oxygen octahedrons, isolating the active centers. XRD analysis indicates the presence of M2 phases. Grasselli et al. reported that the M2 phase is incapable of propane activation due the lack of V5+ sites, but it is a better propylene to acrylic acid catalyst than the M1 phase. During the high propane conversion, the M2 phase begins to serve as a co-catalyst to the M1 phase, converting propylene to acrylic acid [10]. For both Rotavapand Oven-dried catalysts, the presence of the (Mo0.93V0.07)5O14 phase other than the M2 phase does not contribute to the activity of the catalysts. It was reported by Katou et al. that the material with (Mo0.93V0.07)5O14 type structure is much less active in propane oxidation [11].
Figures 6(a) and (b) represent the conversion of propane and selectivity of the partial oxidation products towards the Spray-dried and Rotavap catalysts. As indicated by previous characterization studies, both catalysts out-perform others in propane oxidation. The main products detected are acrylic acid, propylene, carbon monoxide, carbon dioxide and acetic acid. The conversion of propane as a saturated hydrocarbon increases with temperature due to its low reactivity under lower reaction temperatures [12].
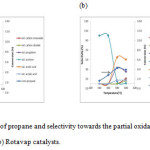 |
Figure 6: Conversion of propane and selectivity towards the partial oxidation products for (a) Spray-dried and (b) Rotavap catalysts. Click here to View figure |
The selectivity of Spray-dried catalysts with acrylic acid increases as the selectivity to propylene decreases with propane conversion. It is believed that acrylic acid is mainly formed from propylene in consecutive reactions. Since the selectivity of COxis low (< 10%) when compared to other main products, it can be concluded that the Spray-dried catalyst is stable. It can perform well under high temperatures and less overoxidation occurs.
Acetic acid is found to be the main product from the reaction with Rotavap catalyst. The selectivity to acetic acid increases when the selectivity to acetone decreases. Therefore, acetic acid is mainly formed from acetone in consecutive reactions. In this case, a hydration mechanism could be involved. However, the possibility of formation of acetic acid from C2 hydrocarbons which might be formed from propane cannot be ruled out [13].
From the product distribution summarized in Table 2, it is clear that both catalysts are active for propane oxidation to acrylic acid. Unfortunately, only the Spray-dried catalyst was found to be selective with acrylic acid. It can be concluded that the sample produced by spray drying is more active and selective than those dried by rotary evaporation.
Table 2: The performance of Spray driedand Rotavapcatalysts for propane oxidation at 400˚C.
|
Catalysts |
Propane Conversion (%) |
Propylene Selectivity (%) |
Acrylic Acid Selectivity (%) |
Acetic Acid Selectivity (%) |
CO2 Selectivity (%) |
CO Selectivity (%) |
|
Spray-dried |
11.6 |
41.1 |
42.8 |
4.1 |
4.2 |
6.7 |
|
Rotavap |
4.1 |
25.3 |
7.3 |
50.4 |
5.9 |
10.5 |
Figure 7 illustrates the changes in propane conversion and selectivity of acrylic acid for both catalysts for the temperature range of 340-400˚C. The temperature of 400˚C produces the best results in terms of the conversion of propane and selectivity of acrylic acid. As previously discussed (Table 2), Spray-driedcatalysts were found to be more active with 11.6% propane conversion, while Rotavap catalystsobtained only 4.1% of propane conversion. Additionally, the selectivity towards acrylic acid for the Spray-dried catalyst(42.8%) is higher than Rotavap (7.3%) which indicates that the Spray-dried catalyst is more selective.
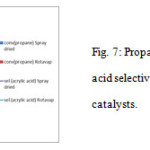 |
Figure 7: Propane conversion and acrylic acid selectivity for the MoVTeNb oxide catalysts. |
Conclusion
The method of drying to obtain a dry catalyst precursor has a pronounced effect on the phase composition of the final MoVTeNb catalyst, which ultimately influences the catalytic properties in propane oxidation. The Spray-dried sample is active and contains two main crystalline phases; orthorhombic M1 and hexagonal M2. The centrifuge process, which is a gravitational method, tends to separate the mother liquid from the precipitate resulting in different metal composition as proven by ICP-AES. The catalysts from rotavap and oven-dried were shown to be ineffective for propane oxidation to acrylic acid.
Acknowledgement
We would like to acknowledge Ministry of Science, Technology and Innovation (MOSTI) for financially supporting this work under the ScienceFund research grant.
References
- Lin M.M., Appl. Catal. A 250: 305-318(2003).
- Lin M.M., Appl. Catal. A 207: 1(2001).
- Solsona B., López Nieto J.M., Oliver J.M. and Gumbau J.P., Catal. Today 91-92: 247-250(2004).
- Ushikubo T., Oshima K., Kayou A. and Hatano M., Stud. Surf. Sci. Catal. 112: 437-480(1997).
- Grasselli R.K., Lugmair C.G. and Volpe-Jr. A.F., Top. Catal. 50: 66-73(2008).
- Ramli I., Botella P., Ivars F., Meng W.P., Zawawi S.M.M., Ahangar H.A., Hernández S. and López Nieto J.M., J. Mol. Catal. A Chem. 342-343: 50-57(2011).
- Evans Jr. H.T., J. Am. Chem. Soc. 90: 3275-3276(1968).
- Sun Y.H., Liu J. and Wang E., Inorg. Chim. Acta. 117: 23-26(1986).
- Popova G.Y., Andrushkevich T.V., Dovlitova L.S., Aleshina G.A., Chesalov Y.A., Ishenko A.V., Playasova L.M., Malakhov V.V. and Khramov M.I., Appl. Catal. A 353: 249-257(2009).
- Grasselli R.K., Catal. Today. 99: 23-31(2005).
- Katou T., Vitry D. and Ueda W., Catal. Today. 91-92: 237-240(2004).
- Zhu B., Li H., Yang W. and Lin L., Catal. Today. 93-95: 229-234(2004).
- Botella P., Solsona B., Martinez-Arias A. and López Nieto J.M., Catal. Letters. 74, 149- 154(2001).
- Grasselli R.K., Buttrey D.J., Santo P.D., Burrington J.D., Lugmair C.G., Volpe A.F. and Weingand T., Catal. Today. 91-92: 251-258(2004).
- Botella P., García-González E., López Nieto J.M. and González-Calbet J.M., Solid State Science. 7: 507-519(2005).
- Beato P., Blume A., Girgsdies F., Jentoft R.E., Schlögl R., Timpe O., Trunschke A., Weinberg G., Basher Q., Hamid F.A., Hamid S.B.A., Omar E. and Mohd Salim L.,
- Appl. Catal. A 307: 137-147(2006).
Fig. 5 shows the temperature-programmed reaction (TPRn) profiles for the three catalysts. A mixture of 5% propane/He gases were passed through MoVTeNbO samples as the temperature increased from 50˚C to 750˚C at a heating rate of 10˚C min-1. The online mass spectrometer continuously recorded signals from propane (m/z=29), CO (m/z=28), propylene (m/z=41), CO2 (m/z=44), acrolein (m/z=56) and (acrylic acid (m/z=72).

This work is licensed under a Creative Commons Attribution 4.0 International License.









