Eco Friendly Procedure for Synthesis of Styrylpyrazoles under Solvent Free Conditions using Microwave Irradiations
Vijender Goel
Department of Chemistry, Maharshi Dayanand University, Rohtak, Haryana, India.
1-(2-Hydroxyphenyl)-5-phenylpent-4- ene-1,3-diones; considerable attention
Download this article as:| Copy the following to cite this article: Goel V. Eco Friendly Procedure for Synthesis of Styrylpyrazoles under Solvent Free Conditions using Microwave Irradiations. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Goel V. Eco Friendly Procedure for Synthesis of Styrylpyrazoles under Solvent Free Conditions using Microwave Irradiations. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25245 |
Introduction
In recent years the solvent free organic reactions assisted by microwaves have gained special attention7,8. The use of microwave irradiations in organic synthesis can increase the purity of resulting products, enhance the chemical yield and shorten the reaction time. Reactions on solid support without using any solvent usually with open vessel in domestic microwave ovens are currently in use for synthetic chemistry to create eco-friendly atmosphere9,10. Solvent free reaction leads to clean, eco-friendly and economic technology. In view of these facts and the interest in microwave assisted organic transformations herein a convenient and efficient method for the synthesis of styrylpyrazoles in solvent free conditions under microwave irradiation using flyash as sold support has been described.
1-(2-hydroxyphenyl)-5-phenylpent-4-ene-1,3-diones obtained by condensation of 2-hydroxyacetophenone with cinnamic acid anhydride under microwave irradiation12 on condensation with phenylhydrazine under microwave irradiation afforded the desired 3- (2- hydroxyphenyl) – 1- phenyl-5styrylpyrazoles in only 90 sec. these compound were characterized on the basis of elemental analysis, IR and NMR spectral data. These styrylpyrazoles were usually obtained by refluxing 1- (2-hydroxyphenyl) -5- phenylpent–4–ene–1,3–diones with phenyl hydrazine in ethanol for 5 hr.13 In the present communication first the diones are obtained in a single pot reaction and then a simple method has been developed for the condensation of these diones with phenyl hydrazine to give styrylpyrazoles under microwave irradiation.
Experimental
Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded on Perkin-Elmer Spectrum BX-series FTIR, 1H NMR spectra on Bruker Avance II 400 MHz NMR spectrometer using tetramethylsilane as internal standard and chemical shift values are reported in δ scale. The reaction was carried out in domestic microwave oven (Samsung, Model No. CE II8 KF, output energy 900W, frequency 2450 MHz) using 30% power for all experiments.
General procedure for microwave assisted synthesis of 3- (2- hydroxyphenyl) –1- phenyl- 5 – styrylpyrazoles .
A mixture of 1- (2-hydroxyphenyl) –5–phenylpent-4–ene–1, 3-dione (5 mmol) and phenyl hydrazine (10 mmol) and the base i.e. triton-B adsorbed on flyash (50% composition) was prepared by adding few drops of acetone, air dried and was subjected to microwave irradiations for 90 sec. Completion of the reaction was checked on TLC and the reaction mixture was dissolved in chloroform. Organic layer was filtered to remove flyash and solvent was distilled off from filterate. The residue was washed with water, dried and recrystalized from methanol to get the desired product.
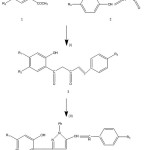 |
Scheme 1 Click here to View scheme |
 
Spectral data of compounds: 4a to 4f
4a: (R1= R2 = R3 = H), m. p 193-940, ( Found C, 81.3; H, 4.6; N, 8.0. C23 H18 ON2 requires C, 81.7; H, 5.3; N, 8.3%). IR (KBr): 3200, 1530, 1480, 1470, 1380, 1350, 1280, 1250. 1140, 1070, 1010, 950, 930. PMR: 6.80 – 7.50 (17H, m, Ar-H and -CH = CH-) and 10.65 (1 H, s, Ar- OH).
4b: (R1 = R3 = H, R2 = CH3), m.p 187-880, (Found C, 81.4, H, 5.3, N, 7.6. C24H20ON2 requires C, 81.8; H, 5.7; N, 8.0%). PMR: 2.30 (3H, s, – CH3), 6.95- 7.60 (16H, m, Ar-H and -CH = CH-) and 10.75 (1 H, s, Ar- OH).
4c: (R1= H, R2= CH3, R3= OCH3), m.p 140-410 (Found: C, 78.2; H, 5.3; N 7.0. C25H22 O2 N2 requires C, 78.5; H, 5.6; N, 7.3%) PMR: 2.33 (3H, s, – CH3), 3.60 (3H, s, OCH3), 6.80 – 7.55 (15H, m, Ar- H and –CH = CH-) and 10.50 (1H, s, Ar-OH)
4d: (R1 = R2 = H, R3 = OCH3), m.p. 143-440, (found : c, 78.0; h, 5.0; N, 7.4. C24 H20O2N2 requires C, 78.3; H, 5.4; N, 7.6%), PMR : 3.79 (3H, s, OCH3); 6.80-7.50 (16H, m, Ar-h and –ch= CH-) and 10.55 (1H, s, Ar-OH)
4e: (R1 = CH3, R2= R3 = H), m.p. 183-840, (Found : C, 81.4 ; H, 5.3; N, 7.8. C24 H20 ON2, requires C, 81.8; H, 5.7; N, 8.0%), PMR : 2.42 (3H, s, CH3), 6.90-7.65 (16 H, m, Ar-H and -CH=CH-) and 10.45 (1H, s, Ar-OH)
4f: (R1 = CH3, R2 = H, R3= OCH3). m.p, 161-620. (found : C, 78.2; H, 5.1; N, 7.0. C25 H22O2N2 requires C, 78.5; H, 5.6; N, 7.3%). PMR : 2.45 (3H, s, CH3), 3.50 (3H, s, OCH3), 6.85-7.55 (15H, m., Ar-H and -CH=CH-) and 10.35 (1H, s, Ar-OH).
Conclusion
The synthetic procedure to get 3- (2- hydroxyphenyl) -1-phenyl-5- styrylpyrazoles using microwave irradiation proved to be highly efficient and convenient method for preparation of this important class of compounds. This method is very much superior to previous conventional heating procedure where the reaction takes much longer time using hazardous solvents and yields are significant lower. It is important to say here that shortening of reaction time and improvement in yields in addition to simplicity of procedures obtained with this method may be of importance for the industrial and large scale production of these compounds.
Acknowledgement
The author is thankful to Maharshi Dayanand University Rohtak, India for providing necessary facilities.
References
- Abadi A.H., Eissa A.A. and Hassan G.S. Chem. Pharm. Bull., 51(7), 838 (2003).
- Jain R., Dixit A. and Pandey P., J. Indian Chem. Soc., 66, 486 (1989).
- Jung J.C., Watkins E.B. and Avery M.A., Heterocyles, 65, 77 (2005).
- Palaska E., Aytemir M., Ozbay T. and Erol D., Eur. J. Med. Chem. 36, 539 (2001).
- Bansal E., Srivastava V.K., and Kumar A., Eur. J. Med Chem. 36, 81 (2001)
- Ahn J.H., Kim H.M., Jung S.H., Kang S.K., Kim K.R., Rhee S.D., Young S.D., Cheon. H.G. and Kim S.S. Bioorg. Med. Chem. Lett., 14, 4461 (2004)
- Caddick S., Tetrahedron, 51, 10403 (1995).
- Loupy A., Petit A., Hamelin J. Jacquault P. and Mathe D. Synthesis, 51, 1213 (1998).
- Loupy A., Petit A., Hamelin J., Barra E.D.D. and Langa F., Synlett, 1259 (1995).
- Varma R.S., Kumar D and Liesen P.J., J. Chem. Soc. Perkin Trans, 1, 4093 (1998).
- Lidstrom P., Tierney J., Wathey B. and Westman J., Tetrahedron, 57, 9225 (2001).
- Goel S., Ritu and Makrandi, J.K., Indian J., Chem., 45 B, 1278 (2006).
- Gaggad, H.L., Wadodkar K.N. and Ghiya, B.J., Indian J. Chem., 24 B, 1244 (1985).

This work is licensed under a Creative Commons Attribution 4.0 International License.









