Cu [(L)Proline]2 As A Novel Catalyst For Synthesis Of 1, 4-Dihydropyridines
Farhad Hatamjafari*
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Article Received on :
Article Accepted on :
Article Published : 19 Oct 2016
We introduce Cu [(L)proline]2 as a Novel Catalyst, cheap, environmentally friendly, and easy separation for Synthesis of 1, 4-Dihydropyridines under solvent-free condition. IR spectra Confirms formation complex of Cu [(L)proline]2.
KEYWORDS:1; 4-dihydropyridines; solvent-free; Cu [(L) proline]2
Download this article as:| Copy the following to cite this article: Hatamjafari F. Cu [(L)Proline]2 As A Novel Catalyst For Synthesis Of 1, 4-Dihydropyridines. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Hatamjafari F. Cu [(L)Proline]2 As A Novel Catalyst For Synthesis Of 1, 4-Dihydropyridines. Available from: http://www.orientjchem.org/?p=22561 |
Introduction
1, 4-Dihydropyridine ring is the common feature for various pharmacological activities such as antitumours1, antivirals2, calcium antagonists3, antidiabetics4, and antagonists5. Recently a number of articles have published on the synthesis of 1, 4-dihydropyridines6-12. Heterogeneous catalysts have gained and have been widely used as a stable and an efficient catalyst for synthesis of organic compound.
Herein, we report an efficient and convenient catalyst for the synthesis of DHPs from ethyl acetoacetate, benzaldehyde, and ammonium acetate using Cu [(L)proline]2 as catalyst (Scheme 1). Once the reaction goes to completion, the catalyst can be filtered, washed with warm ethanol, and reused without decrease in activity.
Previously, we have synthesized a number of heterocyclic compounds13-18. Although many methods are capable of affecting these synthesis but most of the reported are difficult such as separate from the products, long reaction times and low yields. Zn [(L)proline]2 has been used previously as a catalyst for synthesis of organic compound19.
Therefore, we reported the development of an efficient, a facile method, solvent-free and green synthesis for 1,4-DHPs by Cu [(L)proline]2 as catalyst (Scheme 1). There is Cu [(L)proline]2 as the catalyst were cheap, environmentally friendly, and easy separation.
General Procedure for the Preparation of the Cu[(L)proline]2
A mixture of Triethylamine (1 ml) and L-proline (4 mmol) in methanol (10 ml) was added. After solubilization with heat, reaction mixture was stirred for 10 min and Copper (II) acetate (2 mmol) was added. A white precipitate was readily formed and after 45 min it was collected by filtration to give the desired complex. IR spectra confirm formation of Cu [(L)proline]2, IR spectra fig 1 is for L-proline, IR spectra fig 2 is for Copper(II) acetate and IR spectra fig 3 is for Cu [(L)proline]2. Comparison of the spectra shows that loss some of signals and picks is sign for formation complex of Cu [(L)proline]2.
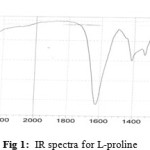 |
Figure 1: IR spectra for L-proline. Click here to View figure |
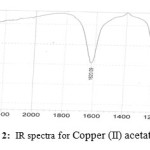 |
Figure 2: IR spectra for Copper (II) acetate Click here to View figure |
![Figure 3: IR spectra for Cu[(L)proline]2](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_-Cu-_Far_fig3-150x150.jpg) |
Figure 3: IR spectra for Cu[(L)proline]2
|
General Procedure for the Preparation of diethyl 2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate
A mixture of ethyl acetoacetate (2 mol), benzaldehyde (1 mol) and ammonium hydroxide (1 mol) and Cu[(L)proline]2 (% 10) in methanol (20 ml was refluxed for 1h. The obtained solid was filtered; the solid was washed with water and recrystallized using absolute ethanol.
Spectral data for diethyl 2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate
Yellow crystals, Yield 94%, IR (KBr, cm-1) ν: 3385, 3044, 2968, 1743. 1H NMR (400MHz, CDCl3) δ: 1.33 (t, 6H, 2CH3, J = 7. 1 Hz), 2.31 ( s, 6H, 2CH3), 4.21 (q, 4H, 2CH2O, J = 7.1 Hz) , 4.87 ( s, 1H, CH), 7.22-7.55(m, 5H, Harom) 8.35 (s, 1H, NH).
![Scheme 1: IR spectra for Cu[(L)proline]2.](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_-Cu-_Far_sch1-150x150.jpg) |
Scheme 1: IR spectra for Cu[(L)proline]2. Click here to View scheme |
Results and Discussion
Herein, we report Cu[(L)proline]2 as catalyst that could provide an efficient, cheap, environmentally friendly, easy separation, high yield, green synthesis, solvent-free and simple route for the synthesis of 1,4-DHPs.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S. and Sakurai Y., Cancer Res., 43: 2905 (1983).
- Krauze A., Germane S., Eberlins O., Sturms I. and Klusa V., Duburs G., Eur. J. Med. Chem., 34: 301 (1999).
- Visentin S., Rolando B., Di Stilo A., Frutterro R., Novara M., Carbone E., Roussel C., Vanthuyne N. and A. Gasco, J. Med. Chem., 47: 2688 (2004).
- Malaise W.J. and Mathias P.C.F., Diabetologia., 28: 153 (1985).
- Poindexter G.S., Bruce M.A., Breitenbucher J.G., Higgins M.A., Sit S.Y., Romine J.L., Martin S.W., Ward S.A., McGovern R.T., Clarke W., Russell J. and Antal-Zimanyi I., Bioorg. Med. Chem., 12: 507 (2004).
- Long, S., Panunzio, M., Petroli, A., Qin, W., and Xia Z., Synthesis., 7: 1071 (2011).
- Vijesh A.M., Isloor A.M., Peethambar S.K., Shivananda K.N., Arulmoli T., and Isloor, N.A., Eur. J. Med. Chem., 46: 5591 (2011).
- Saini A., Kumar S., and Sandhu J.S., J. Sci. Ind. Res., 67: 95 (2008).
- Bhatti R.S., Krishan P., Suresh, and Sandhu, J.S., J. Indian Chem. Soc., 87: 707 (2010).
- Kolvari E., Zolfigol M.A., Koukabi N., and Shirmardi-Shaghasemi B., Chem. Papers., 65: 898 (2011).
- Koukabi N., Kolvari E., Khazaei A., Zolfigol M.A., Shirmardi-Shaghasemi B., and Khavasi H.R., Chem. Commun., 47: 9230 (2011).
- Ladani N.K., Mungra D.C., Patel M.P., and Patel R.G., Chin. Chem. Lett., 22: 1407 (2011).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry., 43: 1349 (2006).
- Hatamjafari F. and Montazeri N., Turkish Journal of Chemistry., 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Rajender Reddya K., Rajasekhara C. V., and Gopi G., Synthetic Communication

This work is licensed under a Creative Commons Attribution 4.0 International License.









