Computational Modeling and Nano-Synthesis of Graphene-Graphite Mixtures for Organic Pollutant Capture from Industrial Water-Drains
A. I. El-Shenawy1,3*, A. H. Atta11,4,, F. A. Kora1,1,5* and Khaled M. Elsabawy2,6
1Department of Chemistry, College of Education, Dammam University, Kingdom of Saudi Arabia.
2Department of Chemistry, Faculty of Science, Taif University, 888 Taif, Kingdom of Saudi Arabia.
3Department of Chemistry, Faculty of Science, Benha University, Benha, Egypt.
4Department of Chemistry, Faculty of Science, Suez University, Suez, Egypt.
5Department of Chemistry, Faculty of Science, Al-Azhar University, Nasr-City, Cairo, Egypt.
6Materials Unit, Chemistry Department, Faculty of Science, Tanta University-31725-Tanta, Egypt.
Computational modeling and visualization studies were performed using computerized advanced programs. The theoretical investigations was succeeded to design Graphene-Graphite mixtures nano-surface catalyst to apply as nano-molecular sieving materials.The synthesized molecular sieving was carefully characterized by both of XRD and AFM to prove the internal structure of the new molecular sieving material. Capture efficiency towards some selected organic herbicides (such as carbaryl pesticide) was examined and investigated in presence of H2O2 which loaded over material surface of G1-G2 (Graphene-Graphite mixture). Hydrogen peroxide was applied as oxidative environmentally friend agent to decompose organic pollutant. The nanosynthesized molecular sieving exhibited very good efficiency towards captures of organic pollutant from industrial water drains such as carbaryl insecticides. Some of the kinetic parameters were investigated in this article, results obtained indicate that, the rate of oxidative degradation of pesticides (carbaryl insecticides) were found to be pH-dependent. The mechanism was proposed and the activation parameters were calculated.
KEYWORDS:G1- G2 = Graphene-Graphite; Pollutant; Carbaryl; Herbicides Modeling
Download this article as:| Copy the following to cite this article: El-Shenawy A. I, Atta A. H, Kora F. A, Elsabawy K. M. Computational Modeling and Nano-Synthesis of Graphene-Graphite Mixtures for Organic Pollutant Capture from Industrial Water-Drains. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: El-Shenawy A. I, Atta A. H, Kora F. A, Elsabawy K. M. Computational Modeling and Nano-Synthesis of Graphene-Graphite Mixtures for Organic Pollutant Capture from Industrial Water-Drains. Available from: http://www.orientjchem.org/?p=11926 |
Introduction
Carbaryl (1-naphthyl methylcarbamate) is a chemical in the carbamate family used chiefly as an insecticide. It is a white crystalline solid commonly sold under the brand name Sevin, a trademark of the Bayer Company. Union Carbide discovered carbaryl and introduced it commercially in 1958. Bayer purchased Aventis Crop Science in 2002, a company that included Union Carbide pesticide operations. It remains the third most-used insecticide in the United States for home gardens, commercial agriculture, and forestry and rangeland protection. Approximately 11 million kilograms were applied to U.S. farm crops in 1976 .
Beltran et al.[1] were investigated the oxidation of atrazine in water by means of direct photolysis at 254 mm and with hydrogen peroxide combined with a u.v radiation. The influence of bicarbonate/ carbonate ions and a commercial humic substance on the oxidation rate has been observed. The oxidation rate is especially fast with the combination of hydrogen peroxide and u.v. radiation.
Technical atrazine and many other pesticides constitute one of the largest groups of organophpsphorous compounds that represent an increasing environmental danger[1, 2]. One of the novel technologies for treating polluted water and wastewater is the advanced oxidation processes (AOPs), by which hydroxyl radicals (·OH) are generated to degrade organic pollutants [3].
Throughout the 20th century, the mechanisms, kinetics, and products of the AOPs using hydrogen peroxide (H2O2), ozone (O3), UV or ultrasonic irradiation, titanium dioxide (TiO2), and Fenton’s reagent, which is a combination of ferrous ions and H2O2, were investigated extensively. These treatments were studied separately or in various combinations [4-7].
Technical Carbaryl and their formulation types have a broad spectrum insecticidal control of sucking and chewing insects, including aphids, house flies, mosquitoes, scale insects and spider mites. Used in fruits, ornamentals, beans, vegetables, and stored products [8].
J.Wang et al. [9] confirm that nanometer rutile titanium oxide powder TiO2 can be used as nano-catalytic degradation of organic pollutants for treating organic waste water.
The essential goals of present article are computational modeling and application of new Graphene-Graphite mixture to apply as nano-molecular sieving materials for oxidative degradation of technical carbaryl organic herbicide .
Experimental
A.Materials Modeling
New series of nano-molecular sieving were designated and visualized using computerized program DIAMOND IMPACT CRYSTAL version 3.2 and MERCURY version 2.3 Germany .The theoretical investigations was succeeded to design new cavity size of Graphene-Graphite mixture see Figs.1,2 to apply as nano-molecular sieving materials .
B. G1-G2 (Graphene-Graphite mixture ) Synthesis
The G1-G2 (Graphene-Graphite mixture ) was prepared by conventional solid state reaction route and sintering procedure using appropriate amount of commercial graphite and ultrapure graphene each purity >99%. The mixture was ground in an agate mortar for one hour. Then the finely ground powder was subject to firing at 870 oC for 5 hours and reground and finally pressed into pellets with thickness 0.15 cm and diameter 1.2 cm. Sintering process was carried out at 1050 oC for 30 hours. Then the furnace is cooled slowly down to room temperature. The brittle pellets were ground and some pieces forwarded to microstructure investigations . Finally the materials are kept in vacuum desiccator .
Carbaryl solutions synthesis
Technical Carbaryl and Atrazine insecticides were supplied from Kafr Elzayat for Pesticides and Chemicals Co., all investigations were performed spectrophotometrically at maximum absorption lMax of Carbaryl which was 280 nm / 412 nm respectively .
Molecular Sever Characterization
X-Ray diffraction (XRD)
The X-ray diffraction measurements (XRD) were carried out at room temperature on the fine ground G1-G2 (Graphene-Graphite mixture ) on the range (2θ =5-70o) using Cu-Kα radiation source and a computerized [Bruker Axs-D8 advance] X-ray diffractometer with two theta scan technique. Analysis of the corresponding 2θ values and the interplanar spacing d (Å) by using computerized program proved that the compound is mainly typical to the visualized one which confirm the quality of preparations .
Atomic Force Microscopic Investigations
Atomic Force Microscopic Investigations (AFM) were carried out using small pieces of prepared samples on different sectors to be the actual molar ratios by using “di-Innova-2009-USA nano-driver” applying tapping mode imaging .The estimated average particle size was calculated and found to be 42.2 nm which reflect how much the preparation route is good .
 |
Graph 1 Click here to View graph |
AFM-tapping mode image recorded for nano-graphite
Kinetic measurements
The kinetic measurements of the reaction were carried out using UV-VIS spectronic 6021 spectrophotometer at λmax. 378 and 412 nm for technical carbaryl pesticide. De-ionized water was used in preparations of all solutions .
Results and Discussion
Modeling of G1-G2 (Graphene-Graphite mixture ) For Sieving Process
New series of nano-molecular sieving were designated and visualized using computerized program DIAMOND IMPACT CRYSTAL version 3.2 and MERCURY version 2.3 Germany .The theoretical investigations was succeeded to design new cavity with inserting ions capability with different sizes of cavities which can apply as nano-molecular sieving materials .
 |
Figure 1: Hexagonal crystal form of Graphite with P63/mmc Space Group . Click here to View figure |
From Figure 1 one can notify that the unit cell of molecular Graphite crystallize as hexagonal crystals and carbons atoms are located in the same crystal sites see also table 1 which describes some selected atomic coordinate positions inside unit cell of crystalline graphite .
Visualizing the crystallographic data of molecular graphite using computerized program DIAMOND IMPACT CRYSTAL version 3.2 and MERCURY version 2.3 Germany gave us the opportunity to judge how much the success of theses mixture as molecular sieving materials see Fig.2 and Table.1 . Fig.2 displays the visualized XRD of hexagonal graphite formulated to apply as molecular sieving materials together with graphene. The characteristics peak of nano graphite lies at ~ two theta = 53.2° as clear in Fig.2b which shifted and located at ~ 48 ° in theoretical visualized pattern as clear in Fig.2a.
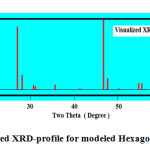 |
Figure 2a: Visualized XRD-profile for modeled Hexagonal Graphite . |
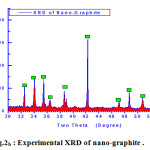 |
Figure 2b: Experimental XRD of nano-graphite . |
Table 1: Selected bond distances and angles inside unit cell of hexagonal Graphite .
|
Atom1 |
Atom2 |
d1-2 Å |
Atom3 |
d1-3 Å |
Angle 213^ |
|
C1 |
C8 |
0.4945 |
C3 |
0.9151 |
50.777 |
|
C8 |
0.4945 |
C8 |
1.0402 |
166.407 |
|
|
C8 |
0.4945 |
C3 |
1.1320 |
24.790 |
|
|
C8 |
0.4945 |
C7 |
1.1977 |
90.954 |
|
|
C8 |
0.4945 |
C7 |
1.2262 |
96.751 |
|
|
C8 |
0.4945 |
C6 |
1.2354 |
50.777 |
|
|
C8 |
0.4945 |
C3 |
1.2812 |
132.564 |
|
|
C8 |
0.4945 |
C6 |
1.4431 |
21.375 |
|
|
C8 |
0.4945 |
C7 |
1.5323 |
53.853 |
|
|
C8 |
0.4945 |
C7 |
1.5689 |
58.303 |
|
|
C8 |
0.4945 |
C6 |
1.5831 |
128.257 |
|
|
C8 |
0.4945 |
C3 |
1.6122 |
86.378 |
|
|
C8 |
0.4945 |
C2 |
1.6658 |
85.716 |
|
|
C8 |
0.4945 |
C3 |
1.6675 |
178.726 |
From table .1 it is clear that there is eight types of carbon atoms symbolized as C1,C2,C3 ,C4 ,C5, C6,C7 and C8 respectively . all carbons atoms are located in the hexagonal structure at the sana distances of bonding even those carbons in the axial positions (C-C) – 0.4945 Å .
Only few angles have some torsion and expected to be unstable as angle ^C1C8C6 = 21.375 ° as clear in table 1 .The majority of angles lie within the normal range of possible torsion which the lattice can resists this torsion without damaging the original hexagonal lattice structure .
Mechanism and order of reaction
The mechanism of oxidative degradation of technical Carbaryl was proposed in our investigations as two step process. The first step, is the fast one which includes the reaction between hydrogen peroxide and Graphene-Graphite solid surface irreversibly with a rate constant K1 (Eq. 1) to form intermediate activated complex C*, but this step is the fast one and irreversible.
The second step is the rate-determining step (slow step) includes the reaction between activated complex C* with the technical Carbaryl with a rate constant K2 (Eq.2).
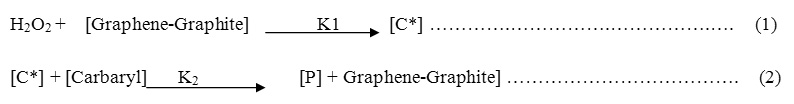
Where [P] is discolored oxidative PESTICIDES , [PESTICIDE] = [Carbaryl] .
The mechanistic sequences may describes as follow: the addition of H2O2 to the catalyst [Graphene-Graphite] surface which includes different oxidation states of carbon (4± δ) we will use symbol M+n which indicate to average oxidation state on the carbon catalyst surface which reacts with H2O2 forming μ2 bound peroxide, which stabilized by hydrogen bonding [10-12] forming activated complex [C*] that finally reacts rapidly reversibly with substrate carbaryl herbicides oxidizing it as described in equation (2).
Order of the reaction
The order of reaction is evaluated by application the conditions of pseudo-first order reaction by keeping H2O2 in large excess and consequently, the overall reaction can be expressed.
![]()
Hence the rate of oxidation depends only on the concentration of
[PESTICIDES] = carbaryl and can be expressed as follows :
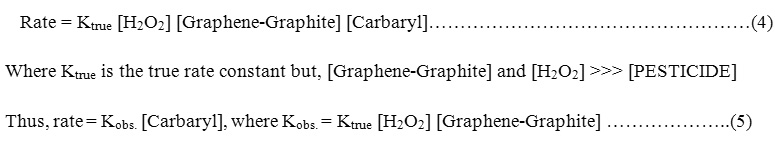
According to the first order reaction condition a plot between in(At – A0) and time was constructed giving straight lines (Fig.3a,b) with slop equal to observed rate constant Kobs. and hence, the true rate constant Ktrue can be easily evaluated by knowing [catalyst] and [H2O2].In this respect, At and A0 are the absorbance of the [PESTICIDE] at time t and infinity, respectively.
![Figure 3a: The first oxidative reaction of [Carbaryl]=3x10-3M with wt of catalysis = 0.02 g, in the presence of H2O2 = 0.04 M, Temp. 28oC and pH = 6.5.](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Comp_Shena_fig3a-150x150.jpg) |
Figure 3a: The first oxidative reaction of [Carbaryl]=3×10-3M with wt of catalysis = 0.02 g, in the presence of H2O2 = 0.04 M, Temp. 28oC and pH = 6.5. |
![Figure 3b: The first oxidative reaction of [Carbaryl]=6x10-3M with wt. of catalysis = 0.02 g, in the presence of H2O2 = 0.04 M, Temp. 28oC and pH = 6.5 .](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Comp_Shena_fig3b-150x150.jpg) |
Figure 3b: The first oxidative reaction of [Carbaryl]=6×10-3M with wt. of catalysis = 0.02 g, in the presence of H2O2 = 0.04 M, Temp. 28oC and pH = 6.5 . |
Efficiency of Graphene-Graphite As Molecular Sieving Material
Fig.4 displays experimental testing of graphene-graphite mixture as molecular sieving trapper for ogranic herbicides under investigation at different conditions of pH-values .It was notified that the efficiency of trapping is remarkably increased at strong acidic (88%) and strong basic medium ( 77% ) which confirms that efficiency of organic herbicide capture ( carbaryl ) is catalysed by both of H+ and OH– that means it could proceeds at acidic or basic conditions .
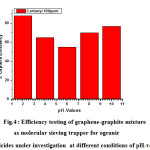 |
Figure 4: Efficiency testing of graphene-graphite mixture as molecular sieving trapper for ogranic herbicides under investigation at different conditions of pH-values . |
Determination of the activation parameters:
Fig.(5) displays the Arrhenius plot of ln K versus 1/T, where T is the absolute temperature K is the observed reaction rate constant at this temperature in accordance with the Eyring- equation [13].
![]()
Where kb and h are the Boltzmann’s and Plank’s constants, respectively.
From this plot, the activation enthalpywas found to be ΔH* = -314.6 KJ/mol & ΔG* = 51.4 KJ/mol and ΔS* = 58.8 J/mol K. These thermodynamic activation parameters help to understand and support the proposed catalytic oxidative mechanism enhancing us to estimate how much the ease of such these reaction to occur spontaneously [15-19] .
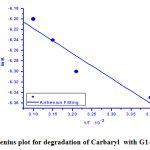 |
Figure 5: Arrhenius plot for degradation of Carbaryl with G1 G2 catalyst . |
The conclusive remarks can be summarized in the following points :
Modeled G1-G2 (Graphene-Graphite nano-mixture) succeeded as molecular siever and environmental catalyst .
Degradation of carbaryl pesticide over G1-G2 (Graphene-Graphite mixture ) is succeeded and proved that the degradation is first order reaction and concentration dependent .
The modeled G1-G2 (Graphene-Graphite mixture ) have different kinds of cavities capable to capture two different sizes of organic pollutants .
The maximum efficiency of trapping for organic pollutant were achieved at strong acidic and strong basic medium which validate and enlarge application of this molecular sieving matter .
Acknowledgements
The authors would like to thank cordially and deeply Dammam University represented by vice president of the university for post graduate studies & researches for their financial support to this research article (2012043).
References
- C. Carvalho, A. Fernandes, H. Pinheiro, I. Goncalves, Chemosphere 67, 7, 1316-1324 (2007).
- A. R. Dincer, Y. Günes, N. Karakaya, J. Hazard. Mater. 141, 3, 529-535 (2007).
- Ku Y., K. Y. Chen, K. C. Lee, Water Res. 31, 4, 929-935 (1997).
- Lapertot M., Ebrahimi S., Dazio S., Rubinelli A., C. Pulgarin, J. Photochem. Photobiol. A 186, 1, 34-40 (2007).
- Irmak S., Kusvuran E., O. Erbatur, Appl. Catal. B 54, 2, 85-91 (2004).
- Song Sh., Ying H. P., He Zh. P., J. M. Chen, Chemosphere 66, 9, 1782-1788 (2007).
- Guo Zh. B., Ch. H. Gu, Zh. Zheng, R. Feng, F. Jiang, G. Zh. Gao, Y. F. Zheng, Ultrason. Sonochem. 13, 6, 487-492 (2006).
- Douglas Hartley ,Agrochemical hand book (1988).
- Wang Jun, Zhijun Pan, Zhaohong Zhang, Xiangdong Zhang, Yuefeng Jiang, Teng Ma, Fuyu Wen, Ying Li, Peng Zhang, Dyes and Pigments, 74, 3, 525-530 (2007).
- Robbins M. H., R. S. Drago, J. Catalysis, 170, 295 (1997).
- Greenwood N., A. Earshaw, “Chemistry of the Elements” Pregmon New York, USA (1989).
- Drago R. S., R. H. Beer, Inorg. Chem. Acta., 198, 359 (1992).
- Nickel U., B. Klein, Ber. Bunsenges, Phys. Chem. 91, 997 (1991).
- Elsabawy Khaled M., Morsy M. A. Sekkinna, Hosny A. El-Daly, M. Jansen, Inorganic Chemistry, 2, 1 (2007).
- Pavan P. C., E. L. Crepaldi, G. D. A. Gomes, J. B. Valim, Colloids; Surfaces A: 154, 399 (1999).
- Cao Y., J. Chen, L. Huang, Y. Wang, Y. Hou, Y. Lu, Journal of Molecular Catalysis A: Chemical, Volume 233, Issues 1-2, 24 May,p 61-66 ( 2005).
- Kaneco S., M. A.Rahman, T. Suzuki, H. Katsumata, K. Ohta ,J. of Photochem. and Photobiology A: Chemistry, Volume 163, Issue 3, ,p. 419-424 (2004).
- Maugans C.B., A. Akgerman, Water Research, Volume 31, Issue 12, Pages 3116-3124 December ( 1997).
- Delgado J.J., J.A. Pérez-Omil, J.M. Rodríguez-Izquierdo, M.A. Cauqui Catalysis Communications, Volume 7, Issue 9, Pages 639-643 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









