A Theoretical Approach to the Study of Some Plant Extracts as Green Corrosion Inhibitor for Mild Steel in Hcl Solution
Ambrish Singh*, Ashish Kumar and Tanay Pramanik
Department of Chemistry, Faculty of Technology and Sciences, SCVE, Lovely Professional University, Phagwara - 144 402, India.
The plant extracts are environmental friendly, biodegradable, nontoxic, cheap and easily available source of material which can successively be used as corrosion inhibitor for mild steel in acid media. The effect of the plant extracts was investigated by theoretical approach. Highest Occupied Molecular Orbital (HOMO), Lowest Unoccupied Molecular Orbital (LUMO), Dipole Moment, Mulliken charges on heteroatoms and Molecular Volume were calculated and interpreted. Quantum chemical calculations revealed the adsorption of molecules onto the surface.
KEYWORDS:Mild Steel; Corrosion inhibition; Plant extract; Quantum Chemical Calculations
Download this article as:| Copy the following to cite this article: Singh A, Kumar A, Pramanik T. A Theoretical Approach to the Study of Some Plant Extracts as Green Corrosion Inhibitor for Mild Steel in Hcl Solution. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Singh A, Kumar A, Pramanik T. A Theoretical Approach to the Study of Some Plant Extracts as Green Corrosion Inhibitor for Mild Steel in Hcl Solution. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25180 |
Introduction
Inhibitors are added to many systems, namely, cooling systems, refinery units, chemicals, oil and gas production units, boiler, and so forth. Most of the effective inhibitors are used to contain heteroatom such as O, N, and S and multiple bonds in their molecules through which they are adsorbed on the metal surface. Plant extracts have become important because they are environmentally acceptable, inexpensive, readily available and renewable sources of materials, and ecologically acceptable. Plant products are organic in nature, and some of the constituents including tannins, organic and amino acids, alkaloids, and pigments are known to exhibit inhibiting action [1]. Moreover, they can be extracted by simple procedures with low cost. Many authors such as E. E. Ebenso, B. Hammouti, A. Y. El Etre, P. C. Okafor, E. Oguzie, and P. B. Raja, have contributed significantly to the green mitigation by investigating several plants and their different body parts as corrosion inhibitors [2-23]. In present work, we have worked on the extracts of (Kalmegh) Andrographis paniculata, (Meethi Neem) Murraya koenigii, (Bael) Aegle marmelos, (Kuchla) Strychnos nuxvomica, (Shahjan) Moringa oleifera, (Orange) Citrus aurantium, and (Arjun) Terminalia arjuna as corrosion inhibitors [24-29]. The active constituents and inhibition efficiencies of the extracts used are shown in Figure 1.
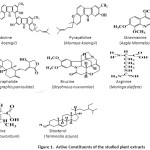 |
Scheme 1 Click here to View scheme |
 
Experimental
Theoretical study
All the calculations were performed with Gaussian 03 for windows. The molecular structures of the species were fully and geometrically optimized using the functional hybrid B3LYP (Becke, three-parameter, Lee-Yang-Parr exchange-correlation function) Density function theory (DFT) formalism with electron basis set 6-31G (*, *) for all atoms. The quantum chemical parameters obtained were EHOMO, ELUMO, EHOMO-LUMO (ΔE), Dipole moment (μ), and Mulliken charges on heteroatoms (O,N,S).
Results and Discussion
Quantum Chemical Calculations
Quantum chemical calculations were carried out in order to investigate adsorption and inhibition mechanism of various inhibitor molecules in the extract. Figure 2 show full geometry optimization of the inhibitor molecules with Mulliken charges. The Frontier molecular orbital (FMO) density distributions of the inhibitor molecules present in Plant extract are shown in Figure 3 (a-b). Frontier orbital theory is useful in predicting adsorption centers of the inhibitor molecules responsible for the interaction with surface atoms. Excellent corrosion inhibitors are usually those compounds who not only offer electrons to unoccupied orbital of the metal, but also accept free electrons from the metal. It is important to focus on the parameters that directly influence the electronic interaction of the inhibitor molecules with the metal surface. These are mainly: EHOMO, ELUMO, ∆E (ELUMO-EHOMO), and dipole moment µ. The values of calculated quantum chemical parameters such as EHOMO, ELUMO, ∆E (ELUMO-EHOMO) and µ of Plant extracts are listed in Table 1. Higher values of the EHOMO facilitate adsorption (and therefore inhibition) by influencing the transport process through the adsorbed layer. Therefore, the energy of the ELUMO indicates the ability of the molecule to accept electrons; hence these are the acceptor states.
The lower the value of ELUMO, the more probable, it is that the molecule would accept electrons. As for the values of ∆E (ELUMO-EHOMO)concern; lower values of the energy difference ∆E will cause higher inhibition efficiency because the energy to remove an electron from the last occupied orbital will be low. For the dipole moment (µ), higher values of µ will favor strong interaction of the inhibitor molecules with metal surface and lower values favor the accumulation of inhibitor molecules around electrode surface. Quantum chemical parameters presented in Table 1 confirmed strong interaction of extracts molecules with the metal surface and thereby forming protective adsorption layer at mild steel/acid solution interface.
Table 1: Calculated Quantum chemical parameters of studied inhibitor.
|
Active Constituents |
HOMO (hartree) |
LUMO (hartree) |
DE (LUMO-HOMO) |
Dipole Moment (µ) |
|
Mahabinine |
-0.15192 |
-0.05321 |
-0.20513 |
11.3843 |
|
Pyrayafoline |
-0.18073 |
-0.02971 |
-0.21044 |
3.9782 |
|
Skimmianine |
-0.12264 |
-0.03787 |
-0.16051 |
1.3965 |
|
Andrographolide |
-0.21563 |
-0.06824 |
-0.28387 |
5.1889 |
|
Threonine |
-0.20226 |
0.00558 |
0.19668 |
1.9030 |
|
Arginine |
-0.05594 |
0.02361 |
0.03233 |
1.0389 |
|
Brucine |
-0.19868 |
0.07845 |
0.12023 |
3.1290 |
|
Sitosterol |
-0.09471 |
0.02543 |
0.06928 |
3.0307 |
 |
Figure 1 Click here to View Figure |
 |
Figure 2Click here to View Figure |
Mechanism of Inhibition
Inhibition performance of Plant extracts for mild steel in 1 M HCl interface depends on the extent of adsorption and adsorption depends on several factors such as the number of adsorption sites, molecular size, and mode of interaction with the metal surface and extent of formation of metallic complexes. The neutral molecules may be adsorbed on the mild steel surface through the chemisorption mechanism, involving the displacement of water molecules from the mild steel surface and the sharing electrons between the hetero atoms and iron. The inhibitor molecules can also adsorb on the mild steel surface on the basis of donor–acceptor interactions between π-electrons of the aromatic ring and vacant d-orbitals of surface iron atoms.
Conclusions
The inhibitors studied had an excellent inhibition effect for the corrosion of mild steel in 1 M HCl. The high inhibition efficiencies of were attributed to the adherent adsorption of the inhibitor molecules on the mild steel surface.
Quantum chemical approach is adequately sufficient to predict the structure and molecule suitability to be an inhibitor.
References
- Ambrish Singh, Eno E. Ebenso, M. A. Quraishi,” Corrosion Inhibition of Carbon Steel in HCl Solution by Some Plant Extracts-A Review”,International Journal of Corrosion, doi:10.1155/2012/897430, 2012.
- A. Y. El-Etre, M. Abdallah, and Z. E. El-Tantawy, “Corrosion inhibition of some metals using lawsonia extract,” Corrosion Science, vol. 47, no. 2, pp. 385–395, 2005.
- E. A. Noor, “Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves,” International Journal of Electrochemcal Science, vol. 2, pp. 996–1017, 2007.
- A. Y. El-Etre, “Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves,” Journal of Colloid and Interface Science, vol. 314, no. 2, pp. 578–583, 2007.
- P. B. Raja and M. G. Sethuraman, “Natural products as corrosion inhibitor for metals in corrosive media—A review,” Materials Letters, vol. 62, no. 1, pp. 113–116, 2008.
- M. Shyamala and A. Arulanantham, “Eclipta alba as corrosion pickling inhibitor on mild steel in hydrochloric acid,” Journal ofMaterials Science and Technology, vol. 25, no. 5, pp. 633–636, 2009.
- A. M. Abdel-Gaber, B. A. Abd-El-Nabey, and M. Saadawy, “The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract,” Corrosion Science, vol. 51, no. 5, pp. 1038–1042, 2009.
- P. Bothi Raja and M. G. Sethuraman, “Solanum tuberosum as an inhibitor of mild steel corrosion in acid media,” Iranian Journal of Chemistry and Chemical Engineering, vol. 28, no. 1, pp. 77–84, 2009.
- M. Lebrini, F. Robert, A. Lecante, and C. Roos, “Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant,” Corrosion Science, vol. 53, no. 2, pp. 687–695, 2011.
- R. Saratha and R. Meenakshi, “Corrosion inhibitor-A plant extract,” Der Pharma Chemica, vol. 2, pp. 287–294, 2010.
- A. Y. El-Etre, “Khillah extract as inhibitor for acid corrosion of SX 316 steel,” Applied Surface Science, vol. 252, no. 24, pp. 8521–8525, 2006.
- M. J. Sanghvi, S. K. Shukla, A. N. Misra, M. R. Padh, and G. N. Mehta, “Inhibition of hydrochloric acid corrosion of mild steel by aid extracts of embilica officianalis, terminalia bellirica and terminalia chebula,” Bulletin of Electrochemistry, vol. 13, no. 8-9, pp. 358–361, 1997.
- E. E. Ebenso, J. Udofot, J. Ekpe, and U. J. Ibok, “Studies on the inhibition of mild steel corrosion by some plant extracts in acidic medium,” Discovery and Innovation, vol. 10, no. 1-2, pp. 52–59, 1998.
- A. Bouyanzer, B. Hammouti, and L. Majidi, “Pennyroyal oil from Mentha pulegium as corrosion inhibitor for steel in 1 M HCl,” Materials Letters, vol. 60, no. 23, pp. 2840–2843, 2006.
- L. R. Chauhan and G. Gunasekaran, “Corrosion inhibition of mild steel by plant extract in dilute HCl medium,” Corrosion Science, vol. 49, no. 3, pp. 1143–1161, 2007.
- E. Khamis and N. Alandis, “Herbs as new type of green inhibitors for acidic corrosion of steel,” Materialwissenschaft und Werkstofftechnik, vol. 33, no. 9, pp. 550–554, 2002.
- H. H. Rehan, “Corrosion control by water-soluble extracts from leaves of economic plants,” Materialwissenschaft und Werkstofftechnik, vol. 34, no. 2, pp. 232–237, 2003.
- M. G. Sethuraman and P. B. Raja, “Corrosion inhibition of mild steel by Datura metel in acidic medium,” Pigment and Resin Technology, vol. 34, no. 6, pp. 327–331, 2005.
- R. A. L. Sathiyanathan, M. M. Essa, S. Maruthamuthu, M. Selvanayagam, and N. Palaniswamy, “Inhibitory effect of Ricinus communis (Castor-oil plant) leaf extract on corrosion of mild steel in low chloride medium,” Journal of the IndianChemical Society, vol. 82, no. 4, pp. 357–359, 2005.
- E. Chaieb, A. Bouyanzer, B. Hammouti, and M. Benkaddour, “Inhibition of the corrosion of steel in 1 M HCl by eugenol derivatives,” Applied Surface Science, vol. 246, no. 1–3, pp. 199–206, 2005.
- P. C. Okafor and E. E. Ebenso, “Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics,” Pigment and Resin Technology, vol. 36, no. 3, pp. 134–140, 2007.
- J. Buchweishaija and G. S. Mhinzi, “Natural products as a source of environmentally friendly corrosion inhibitors: the case of gum exudate from Acacia seyal var. seyal,” Portugaliae Electrochimica Acta, vol. 26, no. 3, pp. 257–265, 2008.
- P. B. Raja and M. G. Sethuraman, “Inhibition of corrosion of mild steel in sulphuric acid medium by Calotropis procera,” Pigment and Resin Technology, vol. 38, no. 1, pp. 33–37, 2009.
- M. A. Quraishi, Ambrish Singh, V.K. Singh, D.K. Yadav, A. K. Singh, ‘’Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves,’’ Materials Chemistry and Physics, 122, 114–122, 2010.
- Ambrish Singh, I. Ahamad, V. K. Singh, M. A. Quraishi, ‘’Inhibition effect of environmentally benign Karanj (Pongamia pinnata) seed extract on corrosion of mild steel in hydrochloric acid solution,” Journal of Solid State Electrochemistry, 15, 1087–1097, 2010.
- Ambrish Singh, V. K. Singh, M. A. Quraishi, ‘’Aqueous Extract of Kalmegh (Andrographis paniculata ) Leaves as Green Inhibitor for Mild Steel in Hydrochloric Acid Solution,” International Journal of Corrosion, doi:10.1155/2010/275983, 2010.
- Ambrish Singh, V. K. Singh, M. A. Quraishi, ’’Effect of fruit extracts of some environmentally benign green corrosion inhibitors on corrosion of mild steel in hydrochloric acid solution,” Journal of Materials and Environmental Science, 1, 162-174, 2010.
- Ambrish Singh, V. K. Singh, M. A. Quraishi,’’Inhibition effect of environmentally benign kuchla (strychnos nuxvomica) seed extract on corrosion of mild steel in hydrochloric acid solution,” Rasayan Journal of Chemistry., 3, 811-824, 2010.
- Ambrish Singh, Ahamad I., V. K. Singh, M. A. Quraishi, ‘’The effect of environmentally benign fruit extract of Shahjan (Moringa oleifera) on the corrosion of mild steel in hydrochloric acid solution,” Chemical Engineering and Communications,doi : 10.1080/00986445.2011.570390, 2011.

This work is licensed under a Creative Commons Attribution 4.0 International License.









