A Facile and Green Synthesis of “5-(3-Methyl-7-Substituted-4h-1, 4-Benzothiazin-2-Yl)-4-Aryl-4h-1, 2, 4-Triazole-3-Thiols” under Ultrasound Irradiation
Bhikan J. Khairnar, Pravin S. Girase and B. R. Chaudhari*
Department of Chemistry, JET’s Z. B. Patil College, Dhule - 424 002, India.
A series of novel 3-methyl-7-substituted-4H-1, 4-benzothiazine-2-carbohydrazide & corresponding thiosemicarbazide have been synthesized. The 1, 4- benzothiazine thiosemicarbazides i.e. 2-[(3-methyl-7-substituted-4H-1, 4-benzothiazin-2-yl) carbonyl]-N-aryl-hydrazine carbothiamide when cyclised with 2N sodium hydroxide via intramolecular dehydrative cyclisation gave benzothiazonyl triazoles. The given procedure is in 4-steps. The final step is an intramolecular cyclisation is achieved by ultrasound irradiation & also by conventional method. In general, substantial improvement in rates and increase in modest yields observed when reactions are carried out under sonication, compared with classical heating method. The structures of these compounds have been elucidated by spectral (IR, 1H NMR) analysis.
KEYWORDS:1,4-Benzothiazine;1,2, 4-triazole; thiosemicarbazide; ultrasonic irradiation
Download this article as:| Copy the following to cite this article: Khairnar B. J, Girase P. S, Chaudhari B. R. A Facile and Green Synthesis of “5-(3-Methyl-7-Substituted-4h-1, 4-Benzothiazin-2-Yl)-4-Aryl-4h-1, 2, 4-Triazole-3-Thiols” under Ultrasound Irradiation. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Khairnar B. J, Girase P. S, Chaudhari B. R. A Facile and Green Synthesis of “5-(3-Methyl-7-Substituted-4h-1, 4-Benzothiazin-2-Yl)-4-Aryl-4h-1, 2, 4-Triazole-3-Thiols” under Ultrasound Irradiation. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25183 |
Introduction
1, 4-Benzothiazine nucleus is found in various drugs, having broad biological spectrum of activities such as anti parasitic, antibacterial, antiviral, antioxidant, anti- inflammatory, anti-hypertensive, anti-anginal drug, antidepressant and anti-tubercular etc. 1-4
The triazole derivative has been found as antithyroid agents, useful for the treatment of gastric ulcer, tuberculastatic activity, tranquillizer & sedative. The heterocyclic systems encompassing 1,2,4-triazole are explored to the maximum extent owing to their wide spectrum of pharmacological activities, such as antitumor, anti- inflammatory, antiviral & CNS-stimulant properties and also have fungicidal, insecticidal, bactericidal & herbicidal activities. 2-6
By considering the above biological activities, it is common observation that combination of two or more biologically active heterocyclic rings either in condensed form or coupled form, results in enhancement of biological profile of such compounds by many folds. In view of this; we are going to synthesize of the novel condensed or coupled derivatives of 1, 4-Benzothiazine & 1, 2, 4-triazole compounds on the basis of an eco- friendly synthetic strategy under ultrasonic irradiation as well as by conventional methods.
An increasing environmental consciousness in chemical research & industry, the challenge for a sustainable environment calls for clean procedure. Ultrasonic assisted organic synthesis as a green synthetic approach is a powerful technique that is being used more and more to accelerate organic reactions. 7-9 It can be extremely efficient and it is applicable to a broad range of practical synthesis. The notable features of ultrasound approach are to enhance reaction rates, formation of purer products in high yields, easier manipulation and considered a processing aid in terms of energy conservation and waste minimization which compared with traditional methods. This technique is more convenient taking green chemistry concepts into accounts. However, the use of ultrasound in heterocyclic system is not fully explored. 10, 11 In order to expand the application of ultrasound in the synthesis of heterocyclic compounds. We wish to report a general, efficient and eco-friendly method for the synthesis of 5-(3-methyl-7-substituted-4H-1, 4-benzothiazin-2-yl)-4-aryl-4H-1, 2, 4-triazole-3-thiols derivatives.
Results and Discussion
The 1, 4 benzothiazine & triazole in combination with derivatives were synthesized and characterized by IR and 1H-NMR spectral analysis in which it complies with the normal values. The melting points of synthesized compounds were determined by open capillary tube method.
In the present work of the experimental procedure for this reaction is remarkably simple and requires no toxic organic solvents or inert atmosphere.
To the best of our knowledge no report is available in the literature using ultrasonic-assisted for this transformation.
Under these novel conditions, it shows the effect of ultrasonic irradiation in these reactions, the synthesis of entitled molecules, with and without ultrasonic irradiation. In all cases the experimental results show that the reaction times are shorter and the yields of the products are higher, under sonication. Based on the results of this study, it seems that the ultrasound irradiation improved the yields of cyclisation product in final step and reduced the reaction time and increases purity of the product.
In the present work the experimental procedure is in four steps and 17 derivatives were synthesized by conventional and ultrasound irradiation method.
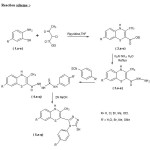
The experimental procedure staring with the synthesis of 2-carboethoxy- 3methyl- 7-substituted-1, 4-benzothiazine-(2a-e) from 2-amino-5-substituted thiophenol (1a-e) with ethyl-α-chloroacetate. In step II, the esters so produced in first step are converted to hydrazide. (3a-e) (3-methyl-7-substitued-1, 4-benzothiazolyl-2-carboxylic acid hydrazide) by hydrazine hydrate and their conversion into 4-aryl-1-(3’-methyl-7’-substituted-2’carbonyl-1,4benzothiazolyl) thiosemicarbazide (4a-q) and in the final step, the intermolecular cyclisation of (4a-q) in 2N NaOH to obtain (5a-q) in good yields by conventional and ultrasonic irradiation methods.
Reaction scheme
Material and Methods
All the starting material was synthesized in laboratory. Melting points were determined in open capillaries in a liquid paraffin bath and are uncorrected. The purity of compounds was checked by TLC. IR spectra were recorded in Nujol on a Perkin-Elmer FT-IR spectrophotometer and PMR spectra in DMSO-d6 using TMS as internal standard.
General Experimental procedure:
Step-I: Preparation of 2-carboethoxy-3-methyl- 7-substituted1, 4-benzothiazine (2a-e).
To a solution of 2-amino-5-substituted thiophenol (1a-e) (0.1 mole) in dry THF; ethyl-α-chloroacetate (0.1mole) was added with stirring followed by the addition of 2-3 drops of pipyridine. The reaction mixture was refluxed for two hours, then it was cooled; solid obtained was filtered and crystallized from ethyl alcohol. The compounds (2a-e) were prepared in the same fashion and their physical constants are given in table-1.
Step II: Preparation of 3-methyl-7-substituted-4H-1, 4- benzothiazine-2-carbohydrazide (3a-e).
To 0.1 mole of (2a-e) in ethanol (20 ml), hydrazine hydrate (0.1 moles, 99%) was added, followed by the addition of a catalytic amount of conc.H2SO4 (2-3drops). The mixture was refluxed for two hours. Excess solvent was removed and on cooling a solid was formed. The solid was crystallized from ethanol. The compounds (3a-e) were prepared in the same method and their physical constants are given in the table-1.
Step III: Preparation of 2-[3-methyl-7-substituted- 4H -1, 4-benzothiazine-2-yl]-N-(aryl) hydrazine carbothiamide. (4a-q).
Above acid hydrazide (3a-e) (0.1 mole) was treated with 4-substitued phenyl isothiocyanate (0.2 mole) in the presence of ethyl alcohol. The reaction mixture was refluxed for 2-hours, cooled, solid obtained was filtered, wash with aq.ethanol and crystallized from ethyl alcohol. The compounds (4a-q) were prepared in the same fashion and their physical constants are given in table-2.
Step IV: Preparation of 5-(3-methyl-7-substituted-4H-1, 4 benzothiazine-2-yl)-4-aryl-4H-1, 2, 4-triazole-3-thiols. (5a-q) by conventional method.
 |
Scheme 1 Click here to View scheme |
The thiosemicarbazide (4-a-q) (0,1mole) was refluxed in NaOH solution (2N) for 3 hours, cooled, poured into water and filtered. The filtrates on acidification with glacial acetic acid gave the crude product (5.a-q) which was filtered, washed with water and crystallized from ethanol.
By Ultrasonic irradiation method:
To a 100 ml round bottom flask was added thiosemicarbazide (4.a-q) (0.01 mol) and 25ml 2N NaOH solution. The reaction vessel was then lowered into a sonication bath and sonicated for 25-40 minutes till a clear solution was obtained. Progress of the reaction was monitored with the help of TLC. After completion of reaction the contents were filtered. The filtrate on acidification gave the crude product (5.a-q) which was filtered, washed with water and crystallized from ethanol.
The comparative data of conventional and ultrasonic irradiation methods for preparation of (5.a-q) is given in table-3.
Table 1: Characterization data of synthesized compounds. (2.a-e) and (3.a-e).
|
Compound No. |
R |
Compound 2 |
Compound 3 |
||
|
Yield (%) |
M.P.in 0C |
Yield (%) |
M.P.in 0C |
||
|
a |
H |
60 |
130 |
60 |
92 |
|
b |
Cl |
82 |
167 |
72 |
190 |
|
c |
Br |
50 |
160 |
65 |
198 |
|
d |
CH3 |
80 |
175 |
70 |
217 |
|
e |
OC2H5 |
45 |
195 |
56 |
240 |
Table 2: Characterization data for the synthesized compounds (4.a-q).
|
Sr. No. |
compound |
R |
R’ |
Yield (%) |
M.P.( 0C) |
|
1 |
4.a |
H |
H |
56 |
138 |
|
2 |
4.b |
H |
Cl |
66 |
216 |
|
3 |
4.c |
H |
Br |
62 |
232 |
|
4 |
4.d |
H |
CH3 |
67 |
170 |
|
5 |
4.e |
H |
OCH3 |
54 |
210 |
|
6 |
4.f |
Cl |
H |
58 |
173 |
|
7 |
4.g |
Cl |
Cl |
64 |
225 |
|
8 |
4.h |
Cl |
Br |
68 |
177 |
|
9 |
4.i |
Cl |
CH3 |
63 |
221 |
|
10 |
4.j |
Cl |
OCH3 |
58 |
232 |
|
11 |
4.k |
CH3 |
H |
56 |
198 |
|
12 |
4.l |
CH3 |
Cl |
61 |
187 |
|
13 |
4.m |
CH3 |
Br |
64 |
176 |
|
14 |
4.n |
CH3 |
CH3 |
65 |
153 |
|
15 |
4.o |
CH3 |
OCH3 |
62 |
194 |
|
16 |
4.p |
Br |
Cl |
56 |
102 |
|
17 |
4.q |
OCH3 |
Cl |
54 |
272 |
Table 3: Characterization data for the synthesized compounds (5.a-q)
| Sr.No. | compound | R | R’ | By conventional method | By ultrasound irradiation | |||
| Yield (%) | M.P.( 0C) | Time (min.) | Yield (%) | M.P.( 0C) | ||||
| 1 | 5.a | H | H | 64 | 145 | 30 | 88 | 145 |
| 2 | 5.b | H | Cl | 72 | 188 | 25 | 90 | 186 |
| 3 | 5.c | H | Br | 70 | 130 | 30 | 90 | 130 |
| 4 | 5.d | H | CH3 | 66 | 194 | 35 | 80 | 192 |
| 5 | 5.e | H | O CH3 | 60 | 224 | 40 | 86 | 226 |
| 6 | 5.f | Cl | H | 74 | 224 | 25 | 92 | 224 |
| 7 | 5.g | Cl | Cl | 72 | 98 | 25 | 85 | 98 |
| 8 | 5.h | Cl | Br | 68 | 102 | 35 | 88 | 102 |
| 9 | 5.i | Cl | CH3 | 64 | 240 | 35 | 86 | 240 |
| 10 | 5.j | Cl | O CH3 | 62 | 255 | 40 | 84 | 256 |
| 11 | 5.k | CH3 | H | 60 | 238 | 30 | 88 | 238 |
| 12 | 5.l | CH3 | Cl | 66 | 254 | 30 | 84 | 252 |
| 13 | 5.m | CH3 | Br | 63 | 197 | 35 | 88 | 200 |
| 14 | 5.n | CH3 | CH3 | 64 | 192 | 35 | 86 | 190 |
| 15 | 5.o | CH3 | O CH3 | 62 | 230 | 40 | 84 | 230 |
| 16 | 5.p | Br | Cl | 58 | 105 | 35 | 86 | 106 |
| 17 | 5.q | OC2H5 | Cl | 56 | 256 | 35 | 88 | 258 |
Conclusion
In conclusion, we have developed a mild and convenient method for the synthesis of entitled compounds. In 25-40 minutes by use of aq.NaOH solution under sonic condition at 40 KHz. We believe that this procedure is better, cost effective, time reducing, and more practical alternative to the existing methods.
Acknowledgement
Authors thank to the Principal, Z.B. Patil College, Dhule & Head (Dept. of Chemistry), Z.B. Patil College, Dhule for providing the lab facilities for this work.
References
- Chaudhari B.R.; Shinde D. B.; Shingare M.S.; Asian Journal of Chemistry. 1995, 07(4), 832-836.
- Ph. D. Thesis of B. R. Chaudhari. Submited to the Dr. Babasaheb Ambedkar Marathwada University, Aurangabad (M.S.).
- Chaudhari B.R.; Shinde D. B.; Shingare M.S.; Indian Journal of Heterocyclic Chemistry. 1995, 04, 187-190.
- Chaudhari B.R.; Shinde D. B.; Shingare M.S.; Polish J. Chemistry. 1996, 70, 1250-1256.
- Holla et al; Indian J. Chem. 2006, 45(B), 2071-2076.
- Narwade S.K., Halnor V. B., Dalvi N. D., Gill C. H., & Karale B.K.. Indian J. Chem. 2006, 45(B), 2776-2780.
- Cravotto G,; Cintas P,; Chem. Soc. Rev. 2006, 35, 180-196.
- Einhorn,C; Einhorn,J. Luche.J;L; Synthesis,1989,787-819
- Mason T.J.; Ultrasonic Sonochemistry, 2003,10,175-179
- Martings M.A.P., Pereira C.M.P., Cunico W., Ultrason. Sonochem. 2006, 13, 364.
- Ji S.J., Wang S.Y., Ultrason Sonochem. 2005, 12,339.

This work is licensed under a Creative Commons Attribution 4.0 International License.









