A DFT Study of Solvation Effects on Tautomerism of 6-oxo Purine by Polarisable Continuum Method (PCM)
Zabialah Heidarnezhad1 *, Fatemeh Heidarnezhad1 , Fatemeh Heydari2 and Elham Bahramian3
1Andimeshk Branch, Islamic Azad University, Andimeshk, Iran. 2Department of Physics, Kermanshah Science and Research, Islamic Azad University, Kermanshah, Iran. 3Shushtar Branch, Islamic Azad University, Shushtar, Iran.
Computational calculations at B3LYP/CC-PVDZ level were employed in the study of tautomers of 6-ox purine (MH) in the gas phase and selected solvents such as benzene, tetrahydrofuran (THF), methanol and water using PCM model. All tautomers are optimized at this level. In addition, stability of the tautomers in different solvents shows interesting results. In the gas phase and benzene (non-polarisable solvent) OP2 form is more stable than the other forms but in polarisable solvents (THF, methanol and water) OP1 is the most stabilized form. Variation of dipole moments and NBO charges on atoms in the solvents were studied.
KEYWORDS:NBO charge; DFT Studies; PCM model; 6-oxo purine; Tautomerism
Download this article as:| Copy the following to cite this article: Heidarnezhad Z, Heidarnezhad F, Heydari F, Bahramian E. A DFT Study of Solvation Effects on Tautomerism of 6-oxo Purine by Polarisable Continuum Method (PCM). Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Heidarnezhad Z, Heidarnezhad F, Heydari F, Bahramian E. A DFT Study of Solvation Effects on Tautomerism of 6-oxo Purine by Polarisable Continuum Method (PCM). Available from: http://www.orientjchem.org/?p=11953 |
Introduction
Tautomerism interconversions 1,2. have been investigated by chemists during last decades. Recently, study of tautomerism received renewed attention due to its importance on the determination of compounds properties and their area of applications. The importance of tautomerism is revealed more since in recent years the investigation about tautomerism has been the major topic in theoretical chemistry. For example, tautomerism in keto-enol 3,4., imine–enamine 5,6., purine 7., pirimidine 8. and many other systems 9. have been studied during the past decades. Thereupon, compounds containing different tautomers can be the subject of interest by theoretical chemists 10. Purine bases and their thio derivatives are found in many biological systems and they are very important from clinical point of view. For example, 6-thiopurine (THIOP) is one of the purine analogues most commonly used in the treatment of the acute human leukaemia 11. It is also known that 6-thiopurine derivatives, among them 2-amino-6- thiopurine has interesting chemotherapy effects, including powerful antitumoral activity 12. The oxopurines are of biological importance, because they are metabolic intermediate products of purine metabolism formed by degradation of nucleic acids. Hypoxanthine (1,7-dihydro-6H-purin-6-one) occasionally occurs as a constituent of the nucleoside inosine in minor amounts in transfer RNA 13 and is oxidized to xanthine and uric acid in man, these reactions being catalyzed by the molybdenum- and iron-containing enzyme xanthine oxidase. Defects in purine metabolism result in an increase in the uric acid level and in the deposition of sodium hydrogen urate monohydrate crystals in joints. This disease, known as gout, is clinically treated by the allopurinol, which has also been used in conjunction with anticancer drugs, combined with 6-mercaptopurine in treatment of leukemia 14,15. Enzymes attack purines at preferred positions and, therefore, the tautomerization of the purine molecules may play an important role in the replication process and spontaneous mutation, and the equilibrium is strongly sensitive to the interaction of these molecules with their environment 16. In equilibrium, oxopurine bases exist in different tautomeric forms, the protropic tautomerism occurring in both rings (pyrimidine and imidazole); thus two types of equilibria can be observed: keto ↔ enol and N(7)H ↔N(9)H. Knowledge of the various geometric and electronic structures as well as the relative stability of tautomeric forms provides a basis for understanding biological activity of oxopurine bases. In addition, knowing how these tautomerization energies change in different environments can provide insight into the influence of solvent effects on molecular stability. The physicochemical properties of the tautomerism of hypoxanthine have been investigated experimentally 17-27 and theoretically 28-32. In a wide variety of environments, including the gas phase, keto tautomers of hypoxan thine are most stable. This conclusion is supported by results of UV 19,21, IR 17,18, Raman 29, 1H NMR19 and 13C NMR 19,23 spectroscopies, and X-ray analysis 24. Pullman and Pullman 28 have investigated the tautomerism of oxopurines by the theoretical CNDO/2 method and three groups of authors 29-31 have reported ab initio quantum mechanical calculations using 6-31G(d), 6-31G(d,p), or MIDI basis sets. Contreras and Alderete 32 used the semiempirical AM1 and MNDO methods to calculate the structure, bondorder matrices, localized MOs, and IR spectra for the tautomers of hypoxanthine in the gas phase.
Computational methods
All these calculations were carried out on a core i7 personal computer by means of GAUSSIAN09 program package. First, all the compounds structures were drawn using Gauss View 03 and optimized in GAUSSIAN09. The tautomers were also optimized in solvents according to the polarisable continuum method of Tomasi and co-workers, which exploits the generating polyhedra procedure 33-37 to build the cavity in the polarisable continuum medium, where the solute is accommodated. Atomic charges in all the structures were obtained using the Natural Population Analysis (NPA) method within the Natural Bond Orbital (NBO) approach. 38
Results and discussion
Gas phase
Structures and numbering of 6-oxo purine are depicted in Figure 1. and the results of energy comparisons of six tautomers in the gas phase and different solvents are given in Table 1. In the gas phase OP2 form is more stable than the other forms. The most and the least differences between OP2 and the other forms in gas phase are found for OP3 amd OP1 with 20.999 kcal mol-1 and 0.302 kcal mol-1 respectively(Table 2). The order of stability of all the tautomers in the gas phase is OP2 > OP1 > OP6> OP5> OP7 >OP4 >OP3. The calculated dipole moments for all forms are presented in Table 3. OP2 tautomer has the smallest dipole moments than the other seven forms with 1.733 D. OP5 tautomer with has the largest dipole moments with 5.0271D in water. The major difference of dipole moment belongs to OP3 form with 3.5453D in gas and water phase. The calculated values NBO charges using the natural population analysis (NPA) of optimized structures of 6-oxo purine tautomers in the gas phase and solvents are listed in Table 3. In OP1 form, N9 atom carries the largest negative charge, in OP1, OP2 nitrogen atoms at position 1 and 9 carry the largest negative charge, carbon atoms at position 2 or 6 carry the largest positive charge and these positions will most effectively interact with nucleophiles.
Solvent effects
Solvent effects are relevant in tautomers stability phenomena, since polarity differences among tautomers can induce significant changes in their relative energies in solution. PCM/B3LYP calculations were used to analyze the solvent effects on tautomerism of 6-oxo purine . It is important to stress that the PCM model does not consider the presence of explicit solvent molecules; hence specific solute–solvent interactions are not described and the calculated solutions effects arise only from mutual solute–solvent electrostatic polarization. The data presented in Table 1. show that polar solvents increase the stability of all 6-oxo purine tautomers in compare to gas phase. The difference between the total energies of 6-oxo purine and the other forms shows a regular trend when changing from gas phase to more polar solvents (water). In solvent and gas non-polarisable phases and benzene OP2 is more stable than other forms, but in polarisable solvents like methanol and water OP1 form is more stable than other forms. The order of stability of all the tautomers in gas phase is OP2 > OP1> OP6> OP5> OP7> OP4 >OP3, and for benzene (non-polarisable solvent) the order of stability is OP2> OP1> OP2> OP4> OP5> OP7 >OP3 and for polarisable solvents (THF, methanol and water the order of stability is OP2> OP1> OP4> OP6> OP5> OP7 >OP3. Total energy shows a regular trend by changing the gas phase to the solution, polarisable solvents increase tautomeric stability compared with gas phase. Based on Table 4. The dipole moments increase by changing the gas phase to the solution as well as by increasing the solvent polarity. The highest dipole moment belongs to OP3 in water solvent by 14.0383 D and the biggest difference of dipole moment is for water anad gas phases by 3.5453 D. The least dipole moment amount for all the forms in all phases belongs to OP2 by 1.7388 D. We have examined the charge distribution of tautomers in the solvent as well as gas phase by using calculated NBO charges. The charge distribution in solvents with increase of polarity differently varies for any atoms.
Conclusion
In the gas phase and benzene (non-polarisable solvent) OP2 form is more stable than the other forms but in polarisable solvents OP1 is the most stabilized form. With increase of polarity total energy of all compounds were more negative.
The charges on all nine positions were affected by solvents. In addition with increase of dielectric constant a variation was found.
The dipole moments of all compounds are affected by solvent. With increase of the polarity of solvents the dipole moments of all tautomers were increased.
Table 1: Total energiesa at B3LYP/6-311++G** in the gas phase and solvents
| Tautomer | Gas(1.0) | Benzene(2.2) | THF(7.6) | Methanol(33) | Water(78.4) |
| OP1 | -487.2231411 | -487.2329258 | -487.239179 | -487.2411882 | -487.2418963 |
| OP2 | -487.2236231 | -487.2330175 | -487.2388823 | -487.2410176 | -487.2413929 |
| OP3 | -487.1901589 | -487.2063456 | -487.2176399 | -487.2220135 | -487.2227977 |
| OP4 | -487.2108888 | -487.2215486 | -487.2283369 | -487.2308381 | -487.2312795 |
| OP5 | -487.2152685 | -487.2223519 | -487.2267134 | -487.2282954 | -487.2285733 |
| OP6 | -487.2171955 | -487.2240913 | -487.2228308 | -487.2298283 | -487.2300949 |
| OP7 | -487.2123809 | -487.2203886 | -487.2255124 | -487.2270985 | -487.2277453 |
aHartree.
Table 2: Energya difirrence between the tautomers of 6-oxo purine
| Tautomer | Gas(1.0) | Benzene(2.2) | THF(7.6) | Methanol(33) | Water(78.4) |
| OP1 | 0.0004820 | 0.0000917 | 0.0000000 | 0.0000000 | 0.0000000 |
| OP2 | 0.0000000 | 0.0000000 | 0.0002967 | 0.0001706 | 0.0005034 |
| OP3 | 0.0334642 | 0.0266719 | 0.0215391 | 0.0191747 | 0.0190986 |
| OP4 | 0.0127343 | 0.0114689 | 0.0108421 | 0.0103501 | 0.0106168 |
| OP5 | 0.0083546 | 0.0106656 | 0.0124656 | 0.0128928 | 0.0133230 |
| OP6 | 0.0064276 | 0.0089262 | 0.0163482 | 0.0113599 | 0.0118014 |
| OP7 | 0.0112422 | 0.0126289 | 0.0136666 | 0.0140897 | 0.014151 |
aHartree.
Table 3: calculated NBO charge on ring atoms of 6-oxo purine
| Tautomer | Atom | Gas | Benzene | THF | Methanol | DMSO | Water |
| OP1 | N1 | -0.6160 | -0.6531 | -0.6506 | -0.6496 | -0.6495 | -0.6495 |
| C2 | 0.3381 | 0.3392 | 0.3438 | 0.3456 | 0.3457 | 0.3459 | |
| N3 | -0.5437 | -0.5737 | -0.5774 | -0.5784 | -0.5785 | -0.5786 | |
| C6 | 0.5231 | 0.6862 | 0.6869 | 0.6870 | 0.6870 | 0.6870 | |
| N7 | -0.2321 | -0.5200 | -0.5374 | -0.5440 | -0.5446 | -0.5452 | |
| C8 | 0.1324 | 0.2591 | 0.2643 | 0.2663 | 0.2665 | 0.2667 | |
| N9 | -0.6732 | -0.5940 | -0.5906 | -0.5892 | -0.5891 | -0.5890 | |
| OP2 | N1 | -0.6242 | -0.6493 | -0.6465 | -0.6454 | -0.6453 | -0.6452 |
| C2 | 0.3329 | 0.3258 | 0.3305 | 0.3323 | 0.3324 | 0.3326 | |
| N3 | -0.4848 | -0.5454 | -0.5587 | -0.5637 | -0.5642 | -0.5646 | |
| C6 | 0.5143 | 0.6698 | 0.6724 | 0.6733 | 0.6734 | 0.6735 | |
| N7 | -0.5902 | -0.5710 | -0.5677 | -0.5664 | -0.5663 | -0.5662 | |
| C8 | 0.1949 | 0.2784 | 0.2831 | 0.2848 | 0.2850 | 0.2851 | |
| N9 | -0.4943 | -0.5502 | -0.5663 | -0.5723 | -0.5729 | -0.5734 | |
| OP3 | N1 | -0.4762 | -0.5930 | -0.6124 | -0.6197 | -0.6203 | -0.6210 |
| C2 | 0.3174 | 0.3338 | 0.3420 | 0.3451 | 0.3454 | 0.3457 | |
| N3 | -0.5343 | -0.6269 | -0.6182 | -0.6146 | -0.6143 | -0.6139 | |
| C6 | 0.5015 | 0.6656 | 0.6664 | 0.6662 | 0.6662 | 0.6662 | |
| N7 | -0.2353 | -0.5106 | -0.5317 | -0.5399 | -0.5406 | -0.5413 | |
| C8 | 0.1169 | 0.2493 | 0.2569 | 0.2600 | 0.2603 | 0.2606 | |
| N9 | -0.5401 | -0.6103 | -0.6032 | -0.6003 | -0.6000 | -0.5998 | |
| OP4 | N1 | -0.4715 | -0.6013 | -0.6173 | -0.6233 | -0.6238 | -0.6243 |
| C2 | 0.3325 | 0.3379 | 0.3434 | 0.3455 | 0.3456 | 0.3458 | |
| N3 | -0.5071 | -0.6090 | -0.6038 | -0.6018 | -0.6016 | -0.6014 | |
| C6 | 0.4967 | 0.6534 | 0.6545 | 0.6546 | 0.6546 | 0.6546 | |
| N7 | -0.5821 | -0.5661 | -0.5646 | -0.5641 | -0.5640 | -0.5640 | |
| C8 | 0.2020 | 0.2781 | 0.2840 | 0.2863 | 0.2866 | 0.2868 | |
| N9 | -0.5616 | -0.5728 | -0.5804 | -0.5828 | -0.5830 | -0.5832 | |
| OP5 | N1 | -0.5251 | -0.5893 | -0.6000 | -0.6041 | -0.6044 | -0.6048 |
| C2 | 0.3186 | 0.3078 | 0.3054 | 0.3043 | 0.3042 | 0.3041 | |
| N3 | -0.5497 | -0.5698 | -0.5731 | -0.5741 | -0.5742 | -0.5743 | |
| C6 | 0.5496 | 0.6251 | 0.6245 | 0.6241 | 0.6241 | 0.6241 | |
| N7 | -0.2799 | -0.5550 | -0.5628 | -0.5655 | -0.5658 | -0.5660 | |
| C8 | 0.1395 | 0.2824 | 0.2889 | 0.2915 | 0.2918 | 0.2920 | |
| N9 | -0.6435 | -0.6051 | -0.6017 | -0.6004 | -0.6003 | -0.6001 | |
| OP6 | N1 | -0.5733 | -0.6226 | -0.6258 | -0.6270 | -0.6271 | -0.6272 |
| C2 | 0.3230 | 0.3093 | 0.3080 | 0.3074 | 0.3074 | 0.3074 | |
| N3 | -0.5478 | -0.5698 | -0.5736 | -0.5748 | -0.5749 | -0.5750 | |
| C6 | 0.5466 | 0.6272 | 0.6264 | 0.6259 | 0.6259 | 0.6258 | |
| N7 | -0.2552 | -0.5310 | -0.5455 | -0.5508 | -0.5513 | -0.5517 | |
| C8 | 0.1338 | 0.2782 | 0.2846 | 0.2870 | 0.2873 | 0.2875 | |
| N9 | -0.6469 | -0.6062 | -0.6025 | -0.6010 | -0.6009 | -0.6008 | |
| OP7 | N1 | -0.5647 | -0.6101 | -0.6135 | -0.6146 | -0.6147 | -0.6148 |
| C2 | 0.3218 | 0.2973 | 0.2960 | 0.2954 | 0.2953 | 0.2953 | |
| N3 | -0.4948 | -0.5417 | -0.5555 | -0.5607 | -0.5612 | -0.5616 | |
| C6 | 0.5341 | 0.6035 | 0.6071 | 0.6086 | 0.6087 | 0.6089 | |
| N7 | -0.5909 | -0.5879 | -0.5827 | -0.5807 | -0.5805 | -0.5803 | |
| C8 | 0.1814 | 0.2939 | 0.3002 | 0.3025 | 0.3027 | 0.3029 | |
| N9 | -0.4985 | -0.5541 | -0.5709 | -0.5772 | -0.5778 | -0.5783 |
Table 4: Calculated dipole moments of optimized tautomers of 6-oxopurine (Deby)
| Tautomer | Gas(1.0) | Benzene(2.2) | THF(7.6) | Methanol(33) | Water(78.4) |
| OP1 | 4.9492 | 5.7059 | 6.2351 | 6.44 | 6.4768 |
| OP2 | 1.7378 | 2.0581 | 2.2887 | 2.3795 | 2.3958 |
| OP3 | 10.439 | 12.209 | 13.4627 | 13.9507 | 14.0383 |
| OP4 | 4.5897 | 5.336 | 5.86 | 6.0652 | 6.1021 |
| OP5 | 4.4289 | 5.1044 | 5.5774 | 5.7607 | 5.7935 |
| OP6 | 2.3234 | 2.727 | 3.0128 | 3.124 | 3.1439 |
| OP7 | 5.0271 | 5.8317 | 6.3951 | 6.6133 | 6.6524 |
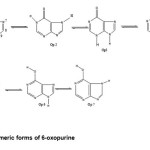 |
Figure 1: Tautomeric forms of 6-oxopurine. Click here to View figure |
References
- Grochowski, J., Serda, P., Markiewicz, M., Kozik, B., Sepiol, J.J., 2004. J. Mol. Struct. 689, 43.
- Belova, N.V., Oberhammer, H., Girichev, G.V., Shlykov, S.A., 2008. J. Phys. Chem. A. 112, 3209.
- Misra, A., Dalai, S., 2007. THEOCHEM 807, 33.
- Zborowski, K., Korenova, A., 2004. THEOCHEM 683, 15.
- Oziminski, W.P., Dobrovolski, J.C., Mazurek, A.P., 2004. THEO- CHEM 680, 107.
- Dines, T.J., Onoh, H., 2006. Spectrochim. Acta A. 64, 891.
- Shukla, M.K., Mishra, S.K., 2000. Comput. J. Chem. 21, 826.
- Bonacin, J.A., Melo, D., Toma, H.E., 2007. Vib. Spectrosc. 44, 133.
- Ralhan, S., Ray, N.K., 2003. THEOCHEM 634, 83.
- Tavakol, H., 2010. THEOCHEM 956, 97.
- M. Hyslop, I. Jardine, J. Pharmacol. Exp. Ther. (1981) 218.
- R. Brockman, Cancer Res. 23 (1963) 1191.
- Hurst, D. T. An Introduction to the Chemistry and Biochemistry of Pyrimidines, Purines and
- Pteridines; John Wiley, New York, 1980, 179–203.
- Rundless, R. W.; Wyngaarden, J. B.; Hitchings, G. H.; Elion, G. B.; Silberman, H. P. Trans. Assoc. Amer. Phys. 1963, 76, 126.
- Elion, G. B.; Callahan, S.; Hitchings, G. H.; Rundles, R. W.; Laszlo, J. Cancer Chemother. Rept. 1962, 16, 1971.
- Scanlan, M. J.; Hillier, I. H. J. Amer. Chem. Soc. 1984, 106, 3837.
- Lin, J.; Yu, C.; Peng, S.; Akiyama, I.; Li, K.; Kao-Lee, L.; LeBreton, P. R. J. Phys. Chem. 1980, 84,1006.
- Sheina, G. C.; Stepanian, S. G.; Radchenko, E. D.; Blagoi, Y. P. J. Mol. Struct. (Theochem.) 1987,158, 275.
- Benoit, R. L.; Frechette, M. Can. J. Chem. 1985, 63, 3053.
- Medeiros, G. C.; Thomas, G. J. J. Biochim. Biophys. Acta 1971, 238.
- Lichtenberg, D.; Bergmann, F.; Neiman, Z. Isr. J. Chem. 1972, 10, 805.
- Psoda, A.: Shugar, D. Biochim. Biophys. Acta 1971, 247, 507.
- Chenon, M. T.; Pugmire, R. J.; Grant, D. M.; Panzica, R. P.; Townsend, L. B. J. Amer. Chem. Soc. 1975, 97, 4636.
- Schmalle, H. W.; Hanggi, G.; Dubler, E. Acta. Crystallogr. 1988, C44, 732.
- Dubler, E.; Hanggi, G.; Schmalle, H. W. Inorg. Chem. 1990, 29,
- Izatt, R. M.; Christensen, J. J.; Ryttine, J. H. Chem. Rev. 1971, 71, 439.
- Tauler, R.; Cid, J. F.; Casassas, E. J. Inorg. Biochem. 1990, 39, 277.
- Pullman, B.; Pullman, A. Advan. Heterocyclic Chem. 1971, 13, 77.
- Hernandez, B.; Luque, F. J.; Orozco, M. J. Org. Chem. 1996, 61, 5964.
- Kassimi, N. El-B.; Thakkar, A. J. J. Mol. Struct. (Theochem.) 1996, 366, 185.
- Nonella, M.; Hanggi, G.; Dubler, E. J. Mol. Struct. (Theochem.) 1993, 279, 173.
- Contreras, J. G.; Alderere, J. B. Mole. Eng. 1992, 2, 29.
- Miertus S, Scrocco E and Tomasi J, J Chem Phys., 1981, 55, 117.
- Cances M T, Mennucci V and Tomasi J, J Chem Phys., 1997, 107, 3032-3037.
- Cossi M, Barone V, Mennucci B and Tomasi J, Chem Phys Lett., 1998, 286, 253-260.
- Barone V, Cossi M and Tomasi J, J Comp Chem., 1998, 19, 404-417.
- Reed A E, Curtiss L A and Wienhold F, Chem Rev., 1988, 88, 899-926.
- Najafi Chermahini A, Nasr-Esfahani M, Dalirnasab Z, Abdol Dabbagh H and Teimouri A, J Mol Struct., (Theochem)., 2007, 820, 7.

This work is licensed under a Creative Commons Attribution 4.0 International License.









