X-ray Diffraction Studies of Co(II), Sm(III) and Nd(III) Complexes with Gliclazide (N-(hexahydrocyclopenta[c] pyrrol-2(1H)-carbamoyl)-4-methylbenzenesulfonamide, An Oral Antidiabetic Drug
Bal Krishan1*, M. Tawkir1 and S.A. Iqbal2
1Department of Chemistry, Safia Science College, Bhopal - 462 001, India. 2Cresent College of Technology Nabi Bagh Karond, Bhopal - 462 038, India.
Article Received on :
Article Accepted on :
Article Published : 22 Oct 2016
Gliclazide(N-hexahydrocyclopentapyrrol-2-carbamoyl)-4-methylbenzenesulphonamide was used to synthesize Co(II),Sm(III) , Nd(III) complexes. Metal complxes were characterized by elemental analysis, IR, NMR,TGA. The crystal structure of complexes were further determined by X-ray diffraction method. The XRD data was used to calculate various parameters like crystal system, volume,density,porosity,particle size etc.which shows that the complexes of Co(II),Sm(III) and Nd(III) are octahedral structure.
KEYWORDS:Gliclazide; Crystal structure; Co(II); Sm(III) and Nd(III) complex
Download this article as:| Copy the following to cite this article: Krishan B, Tawkir M, Iqbal S. A. X-ray Diffraction Studies of Co(II), Sm(III) and Nd(III) Complexes with Gliclazide (N-(hexahydrocyclopenta[c] pyrrol-2(1H)-carbamoyl)-4-methylbenzenesulfonamide, An Oral Antidiabetic Drug. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Krishan B, Tawkir M, Iqbal S. A. X-ray Diffraction Studies of Co(II), Sm(III) and Nd(III) Complexes with Gliclazide (N-(hexahydrocyclopenta[c] pyrrol-2(1H)-carbamoyl)-4-methylbenzenesulfonamide, An Oral Antidiabetic Drug. Available from: http://www.orientjchem.org/?p=22871 |
Introduction
Polyfunctionally rings compounds and synthesis of their metal complex which have various biological activities and include hetero atom, have been formed in organic synthesis and coordination chemistry.1-6 Many trasition and inner trasition metal complexes have been synthesized for analytical and commercial applications many of medicinal use.7-9,24-28 literature survey reveals that the transition and inner transition metal complexes generally crystallized with tetrahedral, octahedral geometry. 10-12
Experimental
All the chemicals used for the preparation of complexes are of Hi-media AR grade E-merk. Metal complexes are synthesized by adding metal salt solution in appropriate solvent to the solution of the ligand. The mixture was refluxed for 3-4 hours. Then the precipitate of metal complxes was obtained. It is filtered, washed and dried in vacuum desiccators.
All selected metals forms 1:2 complexes with gliclazide were confirmed by Jobs method as modified by Turner and Anderson.13-14
Table 1: Physico-chemical and Analytical data of Gliclazide Complexes.
|
01 |
(C15H20N3O3S)2Nd2H2O |
1:2 |
Off White |
45 |
192 |
17.48 (17.33) |
|||||
|
02 |
(C15H20N3O3S)2Co2H2O |
1:2 |
Pink |
56 |
220 |
7.76 (7.76) |
|||||
|
03 |
(C15H20N3O3S)2Sm 2H2O |
1:2 |
Pale Yellow |
53 |
216 |
18.08 (17.96) |
|||||
|
Sr. No. |
% of Carbon observed/ (Required) |
% of H observed/ (Required) |
% N observed/ Required |
% of S observed/ Required |
Stability constant log k lit/mole |
Free Energy Change (-ΔF) |
|||||
|
01 |
43.63 (42.80) |
5.33 (4.86) |
10.18 (9.90) |
7.75 (6.77) |
9.7219 |
-11.7438 |
|||||
|
02 |
48.66 (49.70) |
5.84 (4.86) |
11.35 (7.75) |
8.65 (5.80) |
10.6973 |
-13.7099 |
|||||
|
03 |
43.31
(42.70) |
5.29 (4.86) |
10.10 (9.70) |
7.69 (7.21) |
9.6677 |
-11.6692 |
|||||
Results and Discussion
The X-ray diffraction of Co(II),Sm(III) and Nd (III) complexes with Gliclazide were obtained and summarized in following tables. All reflections has been indexed for h, k, l values using reported literature15-23and full proof suit XRD software v.2.0 by using foolproof suite XRD software the d-values of metal complexes were obtained.
X-ray diffraction study of Gliclazide complexes
The X-ray diffraction pattern of Co(II), Sm(III) and Nd(III) complexes has been determined 2θ range from 6.1963 to 79.97884°,Diffractograms (Fig-1,2,3,) and data has been summarized in the following table..
Gliclazide (GLZ-Co)
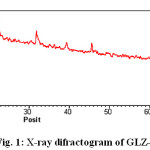 |
Figure 1: X-ray difractogram of GLZ-Co Complex |
Table 1: Cell data and crystal parameter of GLZ-Co complex
a(Å) = 21.6990 Volume(abcsinb)Å= 13880.931
b(Å) = 23.1881 Dcal=4.02100 g/cm3
c(Å) = 27.5891 Dobs= 4.03241 g/cm3
Standard deviation = 0.024% Crystal system = Monoclinic
α=90°, β=89.4°, γ =90° Porosity(%) = 2.837
Density = 0.05329g/cm3 Particle size = 15.794microns
Space group = Pm
|
2θ |
I/I0 |
D(Obs) |
D(Cal) |
h |
k |
l |
|
10.5540 |
69.89 |
8.38237 |
8.46731 |
1 |
0 |
3 |
|
16.3437 |
60.23 |
5.42369 |
5.42470 |
4 |
0 |
0 |
|
19.0109 |
99.40 |
4.66834 |
4.64949 |
2 |
3 |
4 |
|
19.8736 |
82.38 |
4.46760 |
4.46387 |
3 |
4 |
1 |
|
20.4293 |
53.81 |
4.34731 |
4.34770 |
1 |
4 |
4 |
|
22.0439 |
100.00 |
4.03241 |
4.02100 |
5 |
2 |
1 |
|
31.8513 |
52.99 |
2.80964 |
2.80664 |
7 |
1 |
4 |
|
39.3397 |
29.09 |
2.29037 |
2.28905 |
1 |
4 |
11 |
|
45.6199 |
37.40 |
1.98697 |
1.98754 |
9 |
3 |
7 |
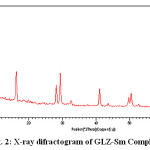 |
Figure 2: X-ray difractogram of GLZ-Sm Complex
|
Table 2: Cell data and crystal parameters for [ (GLZ)2Sm2H2O] complex
a(Å) = 21.7621 Volume (abcsinβ)Å = 14065.269
b(Å) = 23.4271 Dcal = 13.86574 g/cm3
c(Å) = 27.5913 Dobs = 14.17908 g/cm3
Standard deviation = 0.0034% Crystal system = Monoclinic(Octahedral)
α =90°, β=89.2°, γ =90° Porosity(%) = 3.0055 %
Density = 0.059094g/cm3
Space group = Pm Particle size =23.5720 microns
|
2 θ |
I/I0 |
D(Obs) |
D(Cal) |
h |
k |
l |
|
6.2336 |
100 |
14.17908 |
13.86574 |
1 |
1 |
1 |
|
16.2587 |
34.89 |
5.45184 |
5.43999 |
4 |
0 |
0 |
|
28.1979 |
21.43 |
3.16480 |
3.16244 |
-6 |
1 |
4 |
|
29.3552 |
34.62 |
3.04261 |
3.04027 |
2 |
3 |
8 |
|
41.0622 |
19.06 |
2.19818 |
2.19539 |
-4 |
6 |
9 |
|
50.4845 |
14.68 |
1.80782 |
1.80644 |
8 |
3 |
11 |
|
58.0457 |
8.22 |
1.58780 |
1.58759 |
10 |
1 |
12 |
From the cell data and crystal lattice one can conclude that Co(II),Sm(III) and Nd(III) complex is having monoclinic crystal
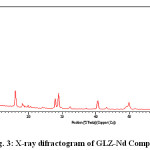 |
Figure 3: X-ray difractogram of GLZ-Nd Complex
|
Table 3: Cell data and crystal parameter of GLZ-Nd Complex
a(Å) = 21.762 Volume (abcsin b)Å= 14065.307
b(Å) = 23.4271 Dcal = 13.86574 g/cm3
c(Å) = 27.274 Dobs =14.26441 g/cm3
Standard deviation = 0.0026% Crystal system = monoclinic
α=90°, β=89.2, γ =90° Porosity(%) = 3.0055
Density = 0.0586592g/cm3 Particle size = 15.484microns
Space group = Pm
|
2θ |
I/I0 |
D(Obs) |
D(Cal) |
h |
k |
l |
|
6.1963 |
100 |
14.26441 |
13.86574 |
1 |
1 |
1 |
|
16.0639 |
16.52 |
5.55751 |
5.51772 |
0 |
0 |
5 |
|
18.2478 |
2.92 |
4.86181 |
4.85591 |
-3 |
1 |
4 |
|
27.9094 |
10.13 |
3.19686 |
3.19271 |
-3 |
5 |
5 |
|
28.9225 |
15.47 |
2.76823 |
3.08474 |
-6 |
3 |
3 |
|
32.3407 |
2.16 |
2.42315 |
2.76573 |
7 |
3 |
3 |
|
37.1028 |
0.92 |
2.22463 |
2.42107 |
4 |
2 |
10 |
|
40.5523 |
8.43 |
2.09735 |
2.22284 |
3 |
10 |
1 |
|
43.1325 |
1.82 |
1.82716 |
2.09549 |
6 |
7 |
7 |
|
49.9132 |
6.76 |
1.76742 |
1.82558 |
-8 |
1 |
11 |
|
51.7224 |
0.86 |
1.61184 |
1.76595 |
12 |
3 |
0 |
|
57.1485 |
2.36 |
1.54030 |
1.61049 |
-6 |
13 |
1 |
|
60.0677
|
0.80
|
1.42229 |
1.53910 |
3 |
9 |
14 |
Conclusion
X-ray diffraction studies also confirms the complexes and formation of new bonds. The number of peaks in Gliclazide are 22 while that of (GLC)2 Nd (GLC)2Co and (GLC)2Sm. are 24,9 and 8 respectively . Thus indicating that complexes formed are a well kit one moreover the X-ray pattern of neither Gliclazide all the reflections present are new ones and the patterns are fairly strong. On comparing the pattern obtained with available literature. It is evident that its pattern is not in good agreement with available information and thus confirms the formation of totally new complexes The X-ray pattern have been indexed by using computer software(FPSUIT 2.0V) and applying interactive trial and error method keeping in mind the characterstics of the various symmetry system,till a good fit was obtained between the observed and the calculated Sin2θ value.The unit cell parameters were calculated from the indexed data, from cell data and crystal lattice parameters of system. (GLC)2Co.2H2O and (GLC)2Sm.2H2O and (GLZ)2Nd2H2O complexes attributed to Monoclinic crystal system .
Table 4
|
Molecular Formula |
complexes |
Molr Weight (gm/mole) |
Crystal/ System |
|
(C15H20N3O3 S)2Co2H2O |
Co(II) |
739.75 |
Monoclinic |
|
(C15H20N3O3 S)2Nd2H2O |
Nd(III) |
825.06 |
Monoclinic |
|
(C15H20N3O3 S)2Sm2H2O |
Sm(III) |
831.18 |
Monoclinic |
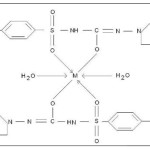 |
Scheme 1
|
Where, M = Co(II),Sm(III) and Nd(III)
Proposed structure of (GLZ)2Co2H2O , (GLZ)2Sm2H2O and (GLZ)2Nd2H2O complexes
Acknowledgement
The author is thankful to the principal of Saifia Science College, Bhopal and Principal, of Cresent College of Technology , Bhopal for providing all necessary facilities and Punjab University for providing XRD spectra.
References
- S.N. Pandeya, D. Sriram, G. Nath and E.De. Clereq, Arzneim-forsch/Drug Res. 50, 55(2000)
- B.S. Hollo, K.A. Poojary and B. Kollurya, II, Farmaco, 51, 793 (1996)
- M. Kidwai, P. Sapra; P. Mishra; R.K. Saxena and M. Singh Bioorg. Med. Chem. 9,217 (2001)
- M.Kumar and S.Ahmad, Orient J,Chem.26(4) 1455-1459(2010)
- H.K. Reddy,D.P.Seshaiah and A.V.Reddy,Orient.J.Chem.27(3);1125-1131(2011)
- B.H. Mehta and S.A. Dugaonkar, Proceeding of 13th, Australia Symposium on analytical chemistry, Darwin Northern, Australia (1995)
- S.A. Dagaonkar, Ph.D. Thesis, University of Mumbai, India (1995)
- R.N.Pandey,D.P.Singh,Priya and R.K.Singh,Orient J.Chem.26(4);1513-1516(2010)
- K.Jamuna D.Harikishore,B.N.Kumar and D.K.Ramana,Orient J.Chem.27(3);1141-1147(2011)
- S. Prakash; Y. Dutt and R.P. Singh: Indian J. Chem, 7, 512 (1969)
- R.P. Bhargava and M. Tyagi, Indian J.Chem.; 25A, 193 (1986)
- N.S. Bhave; R.B. Kharat; J. Chem. Soc. 56, 244 (1979)
- Sharma,R.N.,Sharma,K.P.,and Dixit,S.N.,orient.J.chem.,26(1):283-86(2010)
- Asmi Denavi and Iqbal S.A. Orient J. Chem. 2(2), 156-159 (1986)
- N.F.M. Henry, H. Lipson and W.A. Wooster, Interpretation of X-ray diffraction photograph. 81(1851)
- Chohan, Zahid H.; Praveen M. and Ghaffer A., Synth. React. Inorg. Met. Org. Chem 28(1998), 1973
- Juan Rodriguez – Carvajal and Institute Lue Langevin Diffraction group 6, rue, Jules Horowitz B.P. 156-38042, Greenoble Cedex-9, France/ Email- irc@illfr.
- Altermatt, D. and Brown, I.D. Acta Cryst (1985); B41, 244-247
- Brown, I.D., The Chemical bond in inorganic chemistry, The bond valence model IUCr monographs on crystallography, 12, Oxford University Press, (2002), www.ccp14.ac.uk/ccp/web-mirrors/idbrown
- R.L. Dutta and A. Synmal, Elements of magnetochemistry Affiliated East-West Press Pvt. Ltd. Edn.2(1993)
- A. A. Alhadi S.A.Shaker W.A.Yehe,H.M.Ali and A.A. Mahmood,Orient.J. Chem.,27(4);1437-1442(2011)
- A.S. AL-Janabi,and S.A.Ahmed,Orient.J.Chem.27(4);1563-1571(2011)
- S.Singh,K.K.Singh and J.P. Singh,Orient.J.Chem.27(3);1233-1237(2011)
- M.R.Khan and Sahdev,Orient.J.Chem.27(2);649-653(2011)
- K.Shankar and A.B.Nazeera,Orient.J.Chem.,27(2);655-660(2011)
- Khalil,O.M.,and Reefat,H.M.,Orient.J.chem.,27(9):1581-1590(2011)
- Bhadja,D.R.,Parsania,P.H.,orient.J.chem.,27(4):1699-1707(2011)
- Iqbal,S.A.,and Zaafarny,I.,Orient.J.chem,613-18(2012)

This work is licensed under a Creative Commons Attribution 4.0 International License.









