Synthesis, Structural Characterization and Anti-bacterial Activity of some Novel Schiff base Ligands and their Vanadium (IV) Metal Complexes
G.N. Raja Reddy*, S. Kondaiah, K. Nagaraja Setty, R. Mallikarjuna Rao and J. Sree Ramulu
Department of Chemistry, Srikrishnadevaraya University, Anantapur, Andhra Pradesh, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
A novel Schiff base ligands like OVPTH (p-Toulic Hydrazide and o-Vanilin Schiff base), andOHAPPTH (p-Toulic Hydrazide and o-Hydroxy acetophenone Schiff base) and its vanadium metal complexes have been synthesized and characterized by various physio chemical methods viz. FT-IR, H1 -NMR, UV-Visible, ESR, VSM, Molar conductance, XRD and micro analytical data and found to be anti bacterial activity.
KEYWORDS:Schiffbase; antibacterial activity; characterization; vanadium (IV); OVPTH; OHAPPTH
Download this article as:| Copy the following to cite this article: Reddy G. N. R, Kondaiah S, Setty K. N, Rao R. M, Ramulu J. S. Synthesis, Structural Characterization and Anti-bacterial Activity of some Novel Schiff base Ligands and their Vanadium (IV) Metal Complexes. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Reddy G. N. R, Kondaiah S, Setty K. N, Rao R. M, Ramulu J. S. Synthesis, Structural Characterization and Anti-bacterial Activity of some Novel Schiff base Ligands and their Vanadium (IV) Metal Complexes. Available from: http://www.orientjchem.org/?p=22686 |
Introduction
A large number of Schiff bases and their metal complexes have been found to posses important biological and catalytic activity1-3. These complexes are used as catalysts for water photolysis or reduction of oxygen at a modified carbon cathode. Schiff base have also been used for analytical purposes in the determination of metal ions and some Schiff base derivatives have been used in the extraction of metal solvents. The applications of such complexes depend to a large extent on their molecular structure. Schiff base ligands have an affinity for transition metals such as Cu, Mn, Co and Fe. Some of these complexes have been studied4-5 in great deal for their various structures, steric effects and their coordination chemistry.
The main aim of the present paper is to prepare a new series of metal complexes of VO (IV), with Schiff base ligands derived from p-Toluic hydrazide and different aldehydes and ketones. These complexes were characterized by elemental analysis, IR, NMR, UV, ESR spectroscopy, TG-DTA, Powder XRD, VSM and Conductivity measurements to determine the mode of bonding, geometry and biological activities of the metal complexes6-8 were also studied. These complexes show square pyramidal or trigonal bipyramidal geometry.
Experimental
Materials and Methods
IR spectra were recorded with a Perkin-Elmer IR-598 spectrometer (4000-400 cm-1) using KBr pellets. The 1H NMR spectra of the ligands and their metal complexes are recorded on an AV-400 M-HZ NMR spectrometer in IICT, Hyderabad in DMSO-D6 and CDCl3 solvents at room temperature. The absorbances are measured on Elico SL UV-Visible spectrophotometer. The ESR spectra for all VO (IV) complexes were recorded at room temperature and at liquid nitrogen temperature (LNT) on a Bruker ESP 300E spectrometer in IIT Mumbai. The ESR spectrum obtained from the instrument is a plot of the first derivative of the absorption curve as a function of the magnetic field which was calibrated with an NMR gauss meter and the exact frequency was determined using DPPH radical as a field maker. Magnetic susceptibility data was recorded on an EG and G-155 magneto meter. Thermo gravimetric analyses of the metal complexes were carried out by using the Perken Elmar System in thermal analysis center: STIC KOCHIN.
Synthesis of Schiff base ligands
Synthesis of p-toluic hydrazide and o-Vanilin Schiff base (OVPTH)
Mixture of p-Toluic hydrazide (0.01moles) and o-Vanilin (0.01moles) were dissolved in 50 ml methanol and added few drops of Tri ethyl amine. The whole mixture was transferred in to 250 ml round bottom flask. The mixture was refluxed about one and half hour on water bath. When the reaction mixture was allowed to cool light yellow crystals were obtained. The compound was recrystalized from methanol. The % of yield was 85% and melting point of the compound was 180-1820C.

Scheme 1. Synthesis of p-toluic hydrazide and o-Vanilin Schiff base
Synthesis of p-Toluic hydrazide and o-Hydroxy Acetophenone Schiff base (OHAPPTH)
Mixture of p-Toluic hydrazide (1.50g) and o-Hydroxy Acetophenone (1.36mL) were dissolved in 30 ml methanol and added a pinch of NaOH. The whole mixture was transferred in to 100 ml round bottom flask. The mixture was refluxed about 2 hours on water bath. When the reaction mixture was allowed to cool light brown needles were obtained. The compound was recrystalized from water. The %of yield was 83% and melting point of the compound was 188-190 0C.
Scheme 2. Synthesis of p-Toluic hydrazide and o-Hydroxy Acetophenone Schiff base
Synthesis of Schiff base Metal Complexes
Synthesis of OVPTH Schiff base-VO (IV) metal complex
The reagent p-Toluic hydrazide and o-Vanilin Schiff base (OVPTH) was dissolved in 40ml of methanol and VO (IV) metal ion dissolved in 10ml of distilled water. This mixture was stirred for 6 hours. A colored precipitate of complex was obtained with good yield. The product were washed several times with hot water and cold methanol to free from unreacted metal slats and ligand respectively and finally with ether and dried in vacuo over calcium chloride dessicator.
Synthesis of OHAPPTH Schiff base-VO (IV) metal complex
This metal complex were prepared by adding required amount of Schiff base (2g (0.0074moles)) in 50ml of methanol to the VO(IV) metal ion (0.0074moles) in distilled water in the presence of Sodium acetate and stirred about 10hours. Green precipitates of metal complexe were obtained with good yield. The product were washed several times with hot water and cold methanol to free from unreacted metal slats and ligand respectively and finally with ether and dried in vacuo over calcium chloride dessicator.
Results and Discussion
IR spectral studies
Interpretation of OVPTH ligand and its Vanadium complex
The infrared (IR) spectrum of the compound (OVPTH ligand and OVPTH-VO(IV) complex) is shown in figures 1 & 2.The Infrared spectrum of the ligand is compared with the spectrum of its vanadium complex. The important and specific IR spectral frequencies along with their assignment are given in Table 1.A strong band exhibited at 1645 cm-1 in the IR spectrum of the ligand has been assigned to the (C=N) stretching vibration of the azomethine group. On complexation this band is shifted to 1600cm-1 for OVPTH-VO(IV) complex . Bands appeared at 3215 and 1372 cm-1 due to the stretching and bending vibrations of phenolic OH respectively.9-11 These bands are disappeared in spectra of complexes indicating the deprotanation of phenolic OH. The IR spectrum of the ligand has shown a band in the region 1450-1550 cm-1 due to the C=C stretching vibrations. A broad signal is noticed at 3330 cm-1 in the IR spectrum of the ligand due to N-H stretching vibration. On complexation this band shifted to 3310cm-1 for VO(IV) complex. The IR spectrum of the ligand has shown a sharp band at 1711 cm-1 due to C=O stretching vibration. These results indicate the formation of complex.
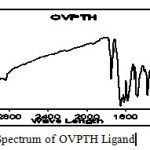 |
fIgure 1:IR Spectrum of OVPTH Ligand Click here to View figure |
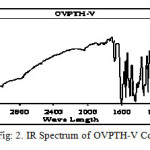 |
figure 2:IR Spectrum of OVPTH-V Complexe Click here to View figure |
Table 1: Important IR Spectral bands of OVPTH and OHAPPTH ligands and its VO (IV) metal complexes
|
Compound |
OH Water |
OH Phenolic |
C=N |
N-H |
C=O |
M-O |
M-N |
|
OVPTH |
– |
3215 |
1645 |
3330 |
1711 |
– |
– |
|
OVPTH-VO (IV) |
3542 |
– |
1600 |
3310 |
1680 |
582 |
486 |
|
OHAPPTH |
3192 |
1654 |
3320 |
1704 |
– |
– |
|
|
OHAPPTH-VO (IV) |
3415 |
– |
1597 |
3331 |
1682 |
633 |
429 |
Interpretation of OHAPPTH ligand and its Vanadium complex
The Infrared spectrum of the OHAPPTH ligand is compared with the spectra of its VO(IV) complex. A strong band exhibited at 1654 cm-1, 3191 cm-1 which was assigned to C=N and Phenolic OH respectively. These bands are disappeared in spectra of complexes indicating the deprotanation of phenolic OH. This is further confirmed by the appearance of new bands in the region of 509 and 648cm-1, which are assigned to the stretching frequencies of M-N and M-O of the metal ligand bands for VO (IV) complex. On complexation this band shifted to 3331 for VO(IV) complex. The IR spectrum of the ligand has shown a sharp band at 1704 cm-1 due to C=O stretching vibration. On complexation this band shifted to 1682cm-1 for VO (IV) complex. These results indicate the formation of complex.
NMR Spectral Studies
Interpretation of NMR spectra of OVPTH ligand and its Vanadium complex
Figs 3 and 4 are shows the NMR spectra of the OVPTH ligand and its VO(IV) complex. Table 2 contains the important chemical shift values along with their assignments. The signals appeared at 8.69, 2.41, 3.81 ppm was assingned to azomethine (H-C=N) proton, methyl protons and methoxy protons respectively. A multiplet is observed in the region 6.8-7.86 due to the aromatic protons. A singlet appeared at 12.04 ppm is attributed to the hydroxyl proton. The singlet appeared at 11.07 ppm due to N-H proton of ligand.
In the 1H NMR spectrum of the OVPTH-VO (IV) complex shows the signals at 8.95, 2.53 and 6.92-7.91 for azomethine proton, methyl protons and aromatic protons respectively.12-15 The singlet appeared at 12.04 ppm due to phenolic hydroxyl proton is absent in the NMR spectrum of VO complex indicating the deprotonation of hydroxyl group and the involvement of that oxygen in coordination.
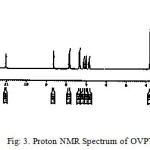 |
Figure 3: Proton NMR Spectrum of OVPTH Ligand Click here to View figure |
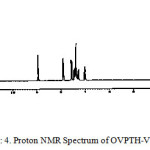 |
figure 4: Proton NMR Spectrum of OVPTH-V Complex Click here to View figure |
Table 2: Important 1H NMR Spectral data of OVPTH and OHAPPTH ligands and its VO (IV) metal complexes
|
Compound |
H-C=N |
CH3-C=N |
Ar-H |
CH3 |
OCH3 |
Ar-OH |
N-H |
|
OVPTH |
8.69 |
6.80-7.86 |
2.4 |
3.81 |
12.04 |
11.07 |
|
|
OVPTH-VO (IV) |
8.95 |
6.92-7.93 |
2.53 |
3.83 |
– |
11.16 |
|
|
OHAPPTH |
– |
2.57 |
6.94-7.89 |
2.41 |
– |
13.51 |
11.25 |
|
OHAPPTH-VO (IV) |
– |
2.63 |
6.93-8.15 |
2.49 |
– |
– |
11.36 |
Interpretation of NMR spectra of OHAPPTH ligand and its Vanadium complex
The 1H NMR spectrum of the OHAPPTH ligand shows signals at 2.57, 2.41 and 6.94-7.89 for methyl protons of azomethine group, methyl protons and aromatic protons of phenyl ring respectively. A singlet appeared at 13.51 ppm is attributed to the hydroxyl proton attached to the phenyl ring in the ligand. The singlet appeared at 11.25 ppm due to N-H proton of ligand.
In the 1H NMR spectrum of the OHAPPTH –VO complex signals are noticed at 2.63, 2.49 and 6.93-8.15 for methyl protons of azomethine group, methyl protons and aromatic protons of phenyl ring respectively. The singlet appeared at 13.51 ppm due to phenolic hydroxyl proton is absent in the NMR spectrum of VO complex indicating the deprotonation of hydroxyl group and the involvement of that oxygen in coordination. A signal observed at 11.25 ppm due to the N-H proton in the ligand is shifted to 11.36 ppm for the VO complex.
UV Spectra
The UV-Visible spectra of the ligands and its metal complexes were recorded in DMF. The electronic spectra of the aqueous solutions of V+4 ions are compared with the corresponding ligands nature. The transitions of these ligands occurred at 303nm, 291nm respectively for OVPTH and OHAPPTH. But on complexation with vanadium metal ion new bands appeared at 410 nm and 405 nm respectively corresponding to the transitional charge transfer from the different ligands to the vanadium metal ion. Bands occurred in the region of 403-410 nm for all complexes are assigned to charge transfer transition (L→M). Based on the results square pyramidal or trigonal bipyramidal structure is proposed for VO (IV) complexes.
ESR Spectral studies
The X-band ESR spectrums of OVPTH-VO(IV) and OHAPPTH-VO(IV) complexes at room temperature shows normal eight lines isotropic features revealing hyperfine splitting of the 51V nucleus I =7/2. The absence of the super hyperfine splitting in this spectrum at room temperature and the trend of g values calculated from the spectrum (gǁ < g⊥< 2) show that the unpaired electron (d1) is in the dxy orbital localized on the metal. The spin Hamiltonian, orbital reduction and bonding parameters of OVPTH-V(IV) and OHAPPTH-VO(IV) complexes are presented in Table 3. Figs 5. Show the ESR spectrum of the OHAPPTH-VO (IV) complex. The values are typical of the spectra displayed by trigonal bipyramidal or square pyramidal VO (IV) complex with one unpaired electron in an orbital of mostly dxy character. For the present OVPTH-VO(IV) and OHAPPTH-VO(IV) complexes G values are greater than four it is 4.07626 and 4.95244 respectively, which suggest that there are no interactions between Vanadium-Vanadium centers in DMF medium. The α2 values of these complexes are 0.637and 0.539 respectively. It indicates that the complex have covalent character. This shows an appreciable covalency in the inplane σ bonding. Spin Hamiltonian Para meters Aǁ* and A⊥* values are obtained from the spectrum (Figure.5) by taking the seventh parts of the magnetic induction in the parallel and perpendicular range from m= +7/2 to -7/2 respectively. Aǁ* >A⊥* is the general trend for trigonal bipyramidal or square pyramidal. The ESR parameters gǁ , g⊥ , Aǁ* and A⊥* of the complexes and the energies of d-d transitions are used to evaluate the orbital reduction parameters ( Kǁ ,K⊥), the bonding parameters ( α2), the dipolar interaction (P). The trend of Kǁ , K⊥values of OVPTH-VO(IV) and OHAPPTH-VO(IV) complexes are Kǁ < K⊥ indicates the presence of out of plane p-bonding Girdano and Bereman suggested the identification16 of bonding groups from the values of dipolar term P. The reduction of P values from the free ion value (0.036cm-1) might be attributable to the strong covalent bonding the value of P obtained for the present complexes are 0.0196 and 0.0199 respectively. The shape of ESR lines, ESR data together with the electronic spectral data suggest trigonal bipyramidal or square pyramidal geometry for OVPTH-VO (IV) andOHAPPTH- VO (IV) complexes.
Table 3: ESR spectral data of OVPTH-VO (IV) and OHAPPTH- VO (IV) complexes
|
Parameters |
OVPTH-VO |
OHAPPTH-VO |
|
g║ |
1.95243 |
1.93126 |
|
g⊥ |
1.98831 |
1.98612 |
|
gave |
1.97635 |
1.96783 |
|
G |
4.07626 |
4.95244 |
|
A║* |
0.01907 |
0.01882 |
|
A⊥* |
0.00664 |
0.00667 |
|
Aave* |
0.01078 |
0.01072 |
|
d-d |
17535 |
17622 |
|
K║ |
0.623 |
0.635 |
|
K⊥ |
0.749 |
0.729 |
|
P* |
0.0196 |
0.0197 |
|
α 2 |
0.637 |
0.539 |
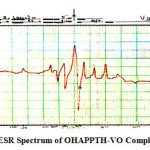 |
figure 5:ESR Spectrum of OHAPPTH-VO Complex Click here to View figure |
Magnetic susceptibility
The magnetic properties of Vanadium complexes help to know the geometry of them. The magnetic properties of these Vanadium complexes are 1.76 B.M. and 1.75 B.M. respectively17-18 for OVPTH-VO(IV) and OHAPPTH- VO (IV). This result clearly indicates that the vanadium complexes of these ligands show square pyramidal or trigonal bi pyramidal geometry.
Molar conductivity
The molar conductance data of complexes which was carried out in DMF at10-3 . The molar conductance values are5.10 and 6.20 ohm-1 cm2mol-1 respectively for VPTH-VO(IV) and OHAPPTH- VO(IV) and RAPPTH- VO(IV). These values suggest non-electrolytic nature of the present complexes.
Thermo gravimetric studies
The Thermo gravimetric studies of all the complexes 19-21were carried out in air at a heating rate of 10o C per minute. The thermo analytical data is summarized in Table.4. The thermal decomposition of the complexes proceeds in three stages. The OVPTH- VO (IV) and OHAPPTH- VO(IV) complexes are thermally stable up to 70.43 and 65.32 oC respectively. The first stage of decomposition corresponding to endothermic dehydration of complexes by the loss of two water molecules occurs in the temperature range 70-120oC and 66-107oC respectively. The intermediates formed are stable up to 251 and 280oc. The second decomposition with exothermic peak by the loss of ligand moiety occurs in the temperature range 251-570 and 280-583oC. The solid residues above 789 and 612 0C were identified as OVPTH-VO(IV) and OHAPPTH-VO(IV) metal oxides respectively. In all the complexes, the final products are metal oxides.
Table 4: Thermal Analytical Data of the OVPTH-VO (IV) and OHAPPTH-VO (IV) vanadium metal complexes
|
Complex X=H2O |
Molecular Weight in grams |
Weight of the complex taken in mgs |
Stage |
Temperature range inoC |
Probable assignment | Mass loss (%) | Total mass loss (%) |
|
VOL22X L=C16 H16 O3N2
|
671.586 |
7.00 |
1 2 3 |
70.43 to 120.29 251.56 to 570.81 Above 789.39 |
Loss of 2H2O molecules
Loss of 2L molecules Corresponds to VO |
5.360 79.17 15.07 |
84.53 |
|
VOL22X L=C16 H16 O2N2 |
637.570 |
7.20 |
1 2 3 |
65.32 to 107.91 280.15 to 583.69 Above 612.59 |
Loss of 2H2O molecules Loss of 2L molecules Corresponds to VO |
5.646 78.213 15.92 |
83.86 |
Table-5..Analytical data of the OVPTH and OHAPPTH ligands and its Vanadium complexes
|
Molecular Formula X=H2O |
Molecular Weight
|
Colour |
Yield in % |
Melting Point in 0C |
Elemental analysis |
|||||||
|
Carbon % |
Hydrogen % |
Nitrogen % |
Metal % |
|||||||||
|
Calc. |
Found |
Calc. |
Found |
Calc. |
Found |
Calc. |
Found |
|||||
| L=C16H16O3N2(OVPTH) |
285.33 |
Light yellow |
85 |
180-182 |
67.29 |
66.78 |
5.60 |
5.35 |
9.81 |
9.52 |
– |
– |
| [VO L2.]2X(OVPTH-VO) |
671.586 |
orange |
69 |
298-312 |
57.17 |
56.49 |
5.06 |
4.67 |
8.39 |
8.09 |
7.58 |
7.03 |
| L=C16 H16O2N2(OHAPPTH) |
268.315 |
Light brown |
85 |
188-190 |
71.55 |
71.21 |
5.96 |
5.42 |
10.43 |
10.02 |
– |
– |
| [VO L2.]2X(OHAPPTH-VO) |
637.57 |
Green |
72 |
302-312 |
60.23 |
59.84 |
5.33 |
4.99 |
8.78 |
7.99 |
7.99 |
7.15 |
The Powder XRD Studies
The powder X-ray diffraction data obtained for OVPTH-VO(IV) and OHAPPTH-VO(IV) complexes with difractograms using DROL-2 powder diffractometer. Radiation filled by metal foil. The difractograms (13 diffractions) reflects Fig.6. between 5-80 (2θ) values for OVPTH-VO(IV) complex and (22 diffractions) for OHAPPTH-VO(IV). Where θ is Brages angle all the main peaks are indicted and calculated values of Miller indices (h k l) along with observed d-specified and reveled intensities are specified in the Fig: 6. All the peaks have been indexed 2θ values compared in graph. Comparison values revels that there is good agreement between values of 2θ and d-values. The powder x-ray diffraction22-23 data showed identical features with very poor crystalinity. The patterns are qualitative and dispersive in intensity for OVPTH-VO(IV) and OHAPPTH-VO(IV) complexes. The XRD patterns are used to explain qualitatively the degree of crystalinity. X-ray Diffraction data of OHAPPTH-VO(IV) complex are presented in Table.6.
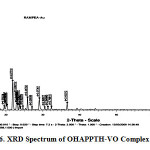 |
figure 6:XRD Spectrum of OHAPPTH-VO Complex Click here to View figure |
Table 6. X-ray Diffraction data of OHAPPTH-VO (IV) complex
|
S.No. |
d expt |
d Calc |
2θ expt |
Calc |
h k l |
|
1 |
7.323 |
7.329 |
12.06248 |
12.073 |
2 1 1 |
|
2 |
14.185 |
14.198 |
6.23856 |
6.244 |
4 2 2 |
|
3 |
14.516 |
14.529 |
6.09709 |
6.103 |
4 2 2 |
|
4 |
15.607 |
15.621 |
5.67325 |
5.678 |
4 3 2 |
|
5 |
16.899 |
16.915 |
5.24221 |
5.247 |
4 4 1 |
|
6 |
18.037 |
18.054 |
4.91407 |
4.919 |
5 3 2 |
|
7 |
19.493 |
19.511 |
4.55014 |
4.554 |
5 3 3 |
|
8 |
20.038 |
20.057 |
4.42757 |
4.432 |
6 3 1 |
|
9 |
20.256 |
20.275 |
4.38047 |
4.384 |
6 3 1 |
|
10 |
21.781 |
21.801 |
4.0771 |
4.081 |
5 5 2 |
|
11 |
22.856 |
22.877 |
3.88772 |
3.891 |
5 5 3 |
|
12 |
23.089 |
23.110 |
3.84904 |
3.853 |
6 4 3 |
|
13 |
23.599 |
23.621 |
3.76691 |
3.770 |
6 5 1 |
|
14 |
23.881 |
23.903 |
3.72321 |
3.727 |
6 2 1 |
|
15 |
24.558 |
24.580 |
3.62203 |
3.625 |
6 5 3 |
|
16 |
25.826 |
25.850 |
3.44702 |
3.450 |
6 6 2 |
|
17 |
27.036 |
27.061 |
3.29536 |
3.298 |
7 5 3 |
|
18 |
29.028 |
29.055 |
3.07361 |
3.076 |
7 6 3 |
|
19 |
30.498 |
30.526 |
2.92877 |
2.931 |
8 6 2 |
|
20 |
31.368 |
31.397 |
2.84945 |
2.852 |
7 6 5 |
|
21 |
32.314 |
32.344 |
2.76815 |
2.771 |
8 6 3 |
|
22 |
36.617 |
36.652 |
2.45212 |
2.454 |
8 7 6 |
Antibacterial Activity of OVPTH and OHAPPTH ligands and its vanadium (IV) Metal Complexes
Paper disc diffusion technique
The sterilized (autoclaved at 120ºC for 30 min) medium (40–50ºC) was inoculated (1 ml/100 ml of medium) with the suspension (5X10-5 cfu/ml) of the microorganism (matched to McFarland barium sulphate standard) and poured into a petridish to give a depth of 3–4 mm. The paper impregnated with the test compounds (100 μg/ml in dimethyl formamide) was placed on the solidified medium. The plates were pre-incubated for 1 h at room temperature and incubated at 37ºC for 24 and 48 h for antibacterial and antifungal activities, respectively. Streptomycin (100 μg/ml/disc) was used as standard for anti-bacterial activitiy. A comparison of antibacterial activity of the synthesized compounds with that of standard drugs was effectively24-27 presented in Table 7. In this study, all the synthesized compounds were evaluated for their in vitro antibacterial activity against two bacterias namely E.coli and B. subtiles. Streptomycin was used as a reference drug. The antibacterial activity results indicate that most of the metal chelates exhibited good to moderate antibacterial activity when compared to streptomycin. Among the synthesized metal complexes OHAPPTH-V0 (IV) showed high activity.
 |
Figure 7: VPTH-VO(IV) Complex Click here to View figure |
 |
figure 8: OHAPPTH-VO(IV) Complex Click here to View figure |
Table 7 : Antibacterial activities of ligands and their transitions metal complexes (Zone of inhibition in mm)
|
Compound |
E.Coli |
B. Subtiles |
| OVPTH ligand |
7 |
6 |
| OVPTH-VO (IV) |
11 |
8 |
| OHAPPTH ligand |
6 |
6 |
| OHAPPTH-VO (IV) |
12 |
9 |
| Streptomycin |
22 |
24 |
Conclusion
In the present research studies, our efforts are synthesized of some newly compounds. These synthesized compounds characterized by various physio- chemical and spectral analysis. By using above all spectral studies it is concluded that they behave as bidentates during complexation. The IR data of both Schiff base and its vanadium metal complexes show that the OVPTH andOHAPPTH Schiff bases are coordinate to the metal ion in bidentate manner with ON donor sites of azomethaine nitrogen and phenolic oxygen. The ESR spectras and data suggest trigonal bi pyramidal or square pyramidal structures for OVPTH-VO(IV) and OHAPPTH-VO(IV) Complexes. On the basis of chelation theory, metal complexes have more biological activity than free ligands.
References
- Bensaber, S.M. Mailub A.A., Hudere S.S. et al., (2005) Micro. chem. J. 81, 191-194.
- El-tajoury, A.N. M.M. El-ajaily, A.A. Maihub, S.F. Ben Geweirif, Pure & applied Journal, Sebha University, 5(1) 108-123 (2006).
- Maihub, A.A. M.M. El-ajaily, S.M. Filog, Al-A bhath Al-Yarmouk Journal, 14(1), 119- 128 (2005).
- Mishra, A.P., Pandey, L.R., 2005. Indian J. Chem. 44A, 1800.
- El-ajaily, M.M. A.A. Maihub, M. Aboukrisha, A.I. Salem, Jerash for Researches & Studies, Part I, 6(2), 7-20 (2002).
- Jarbou, A. M. M. Sc Thesis. Garyounis University (2006).
- Chandra S. and U. Kumar, Spectrochim. Acta, 61A: 269-275 (2005)
- Sharma, K. R. Singh, N. Fahmi and R.V. Singh, Spectrochim. Acta, 75A: 422-427(2010).
- Dubey, R.K. U.K. Dubey and C.M. Mishra, Indian J. Chem., 47: 1208-1212 (2008).
- Nakamoto, K. Infra red and Raman spectra of Inorganic and coordination compounds, 11. 5th ed. John Wiley, Sons, Part A, B, New York,1998
- Mishra, A.P, R.K. Mishra and S.P. Srivastava, J. Serb. Chem. Soc. 74: 523-535 (2009).
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed. Elsevier, New York, 1984.
- Mishra A.P, and L.R. Pandey, Indian J. Chem., 44A: 94-97 (2005).
- Mohamed, G.G. M.M. Omar and A.A. Ibrahim, Eur. J. Med. Chem., 44: 4801-4812(2009).
- Mukherjee A, Dhar S, Nethaji M, Chakravarty AR, 2005. Journal of the Chemical Society Dalton Transactions, 349-353.
- Suberu H, 2004. African Journal of Biotechnology,3: 468–472.
- Adhikary C, Mal D, Okamoto KI, Chaudhuri S, Koner S, 2006. Polyhedron, 25: 2191- 2197.
- Dong, Y; Narla, RK; Sudbeck, E; (2002) Vol. 78, pp 321-330.
- Sonmez, M. and Sekerel, M. (2002). Pub. J. of chem., 76, 907-914.
- Prabhu, PS and Dodwad S.S. (1986). J. Indian of chem., 58, 478-482.
- Sujamol, M.S., Athira, C.J., Sindhu, Y., Mohanan, K., 2010. Spectrochim. Acta 75A,106.
- Gupta S.K. Hitchcock and Kushwah Y.S. (2002), J. of coord. Chem…, 55 (12), 1401-1407.
- Kralova K, Kissova K, Svajlenova O and Vanco J, Chem. Pap., 2004, 58(5), 361.
- Parekh J, Inamdhar P, Nair R, Baluja S and Chanda S, J. Serb.Chem., Soc., 2005, 70, 1161
- Vaghasia Y, Nair R, Soni M, Baluja S and Chanda S, J. Serb. Chem. Soc. 2004, 69, 991.
- Raman N, Res. J. Chem. Environ., 2005, 9(4).

This work is licensed under a Creative Commons Attribution 4.0 International License.










