Synthesis of Some New Heterocyclic Nitrogen Compounds Starting from Pyromellitic Dianhydride
Ahmed M. Abo-Bakr1 *, Mamdouh A. Hassan2 , Husien. H. Temirek1 and Ahmed M. Mosallam1
1Department of Chemistry, Faculty of Science, South Valley University, Qena, Egypt.
2Pharmaceutical Chemistry Department, Faculty of Pharmacy and Pharmaceutical Industries, Sinai University, North Sinai, Egypt.
Article Received on :
Article Accepted on :
Article Published : 01 Dec 2012
Pyromellitic dianhydride 1 was used as starting compound for the synthesis of some new derivatives of condensed dipyrrole, dibenzoxazine, and dipyridazine. Thus, the diimide 2 was formed on fusion of 1 with urea, thiourea and/or thiosemicarbazide. Also, 1 reacted with benzylamine to give terephthalic acid derivative 3 which on fusion afforded the cyclic diimide 4. The reaction of 1 with o-aminothiophenol under different reaction conditions was investigated to give 5 in acetic acid or 6 in toluene and the later could be decarboxylated to 7. On the other hand, the action of AlCl3 on 1 in presence of reactive aromatic substrates afforded the corresponding isomers 8a-d and 9a-d. which could be cyclized using hydroxylamine hydrochloride to give the dioxazine isomers 10a-d and 11a-d. The dioxazine isomers 10b and 11b were also obtained when 14 was allowed to react with AlCl3 in anisol. Cyclization of 8a-d and/or 9a-d using hydrazine or phenylhydrazine gives the dipyridazine isomers 13a-h and/or 14a-f respectively.
KEYWORDS:Pyromellitic dianhydride; Pyromellitimide; Dipyridazines; Dibenzoxazines; Dipyrroles
Download this article as:| Copy the following to cite this article: Abo-Bakr A. M, Hassan M. A, Temirek H. H, Mosallam A. M. Synthesis of Some New Heterocyclic Nitrogen Compounds Starting from Pyromellitic Dianhydride. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Abo-Bakr A. M, Hassan M. A, Temirek H. H, Mosallam A. M. Synthesis of Some New Heterocyclic Nitrogen Compounds Starting from Pyromellitic Dianhydride. Available from: http://www.orientjchem.org/?p=11899 |
Introduction
Owing to the wide spread applications of pyromellitic dianhydride (PMDA) in several fields, such as synthesis of polyimides [1-3], epoxy resins [4] and Metal Carboxylate [5,6] Complexes. In addition, pyrrole, benzoxazine and phthalazine derivatives exhibit wide range of pharmacological and biological applications, such as analgesic [7-9], antifungal [10-12], antitoxic [13], anticancer [14-17], alkaloids, agro-chemicals [18,19] and dyes applications [20], this encourage us to synthesize new derivatives of condensed dipyrrole, dibenzoxazine and dipyridazine starting with pyromellitic dianhydride which may posses a greater certain pharmacological activity.
Results and discussion
During the last few years our research group has been interested in the chemistry of anhydrides with the objective of finding new routes for the synthesis of new heterocyclic derivatives with expected biological activities [21-24].
E.V.Ganin et al [25], were able to fined a synthetic procedure for the preparation of pyromellitic diimide 2 by the reaction of PMDA 1 with formamide. In our work, we have investigated the action of other amides such as urea, thiourea, and thiosemicarbazide on the anhydride 1 in order to synthesize new diimide derivatives which may undergo cyclization or further polymerization, the only product isolated from these reactions was pyromellitic diimide 2 in good yield (cf. Scheme 1).
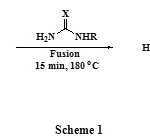 |
Scheme 1 |
When compound 1 was allowed to react with benzylamine in dry toluene, 2,5-di[(benzylamino)carbonyl]terephthalic acid 3 was formed through cross linked nucleophilic attack. The presence of electron withdrawing carbonyl group in para position to the anhydride carbonyl carbones facilitates the cross nucleophilic attack [26]. Fusion of compound 3 gave the corresponding cyclic diimide assigned as 2,6-dibenzylpyrroloisoindole-1,3,5,7-tetrone 4 (cf.Scheme 2).
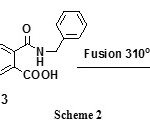 |
Scheme 2 |
PMDA was reacted with 2-aminothiophenol in glacial acetic acid as a proteic solvent to give 2,5-di(benz-1,3-thiazol-2-yl)terephthalic acid 5 through cross nucleophilic ring opening of PMDA followed by intramolecular nucleophilic cyclization to give benzothiazole moiety. Repeating the reaction in non-polar solvent namely, toluene gave 2,5-di(benz-1,3-thiazol-2-yl)benzoic acid 6 which on thermal decarboxylation at 320oC gave 1,4-di(benz-1,3-thiazole)benzene 7 via losses of CO2 (cf. Scheme3).
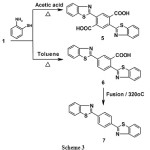 |
Scheme 3 |
The reaction of 1 to give compound 6 may proceed via losses of CO2 through the following intermediate shown in
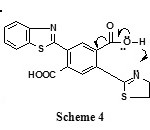 |
Scheme 4 |
The molecular ion peak of compound 6 indicated (M+·) at m/z = 388 (9.2%) corresponding to the formula C21H12N2O2S2, and the following fragents observed in the mass spectrum of the compound 6 confirms its assigned structure (Scheme 5): The peaks at 387, 344, 343, 311, and 235 are characteristic peaks corresponding to the fragments. Thus the structure of compound 6 is in agreement with the observed spectral data.
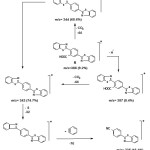 |
Scheme 5 |
On the other hand, the Friedel-Crafts reaction of pyromellitic dianhydride with benzene to give 2,5-dibenzoylterephthalic acid 8a and its isomer 4,6-dibenzoyl- isophtalic acid 9a was previously described [27]. In our work, the same reaction was intensively investigated using more reactive aromatic substrates namely anisol, toluene in addition to chlorobenzene.
The two isomeric structures formed in each reaction were separated by fractional crystallization to give 2,5-diaroylterephthalic acid 8a-d and 2,6-diaroylisophthalic acid 9a-d (Scheme 6). The configuration assigned to these proposed structures was based on the IR spectroscopic evidence. 1H-NMR for terephthalic isomers 8a-dindicates the presence of a singlet for the two identical benzene protons at δ 7.88-7.92, while for isophthalic isomers 9a-d showed two different benzene protons at δ7.31-7.95 and at δ 8.55-8.77 respectively.
2,5-diaroylterephthalic acids 8a-d and/or 2,6-diaroylisophthalic acids 9a-d were reacted with hydroxylamine hydrochloride in pyridine under reflux, compounds 10a-d identified as 4,9-diaryl-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-diones and/or 11a-d identified as 4,6-diaryl-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-diones were obtained respectively (Scheme 6).
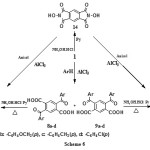 |
Scheme 6 |
The H-C COSY technique (Carbon-13 detection and proton decoupling) is attractive because it efficient and provides unequivocal results; it allows the shift of two nuclei (1H and 13C) to be measured in a single experiment. Figures (1) and (2) showed H-C COSY for the two isomers 8d and 9d in which the shifts of the 1H and 13C nuclei are bonded to one another are read as coordinates of the cross signal. In (Fig. 1), for example, the protons with shifts 7.6 and 7.96 are bonded to the carbon atoms at 128.91 and 128.82 respectively for compound 8d. On the other hand, compound 9d, the protons with shifts 7.58 and 7.95 are bonded to the carbon atoms C-2 and C-9 at 128.84, while the protons with shifts 7.74 and 8.54 are bonded to the carbon atoms C-3 and C-10 at 130.67 and 131.38 respectively (cf. Fig. 2).
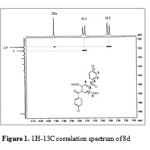 |
Figure 1: 1H-13C correlation spectrum of 8d |
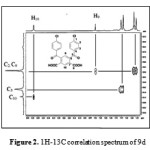 |
Figure 2: 1H-13C correlation spectrum of 9d |
13C-NMR data were also useful to differentiate between the two isomers 8d and 9d in which compound 8d showed nine different signals for nine non identical carbon atoms, while for 9d showed ten different carbon atoms.
Through another synthetic route, the two isomeric structures 10b and 11b were also obtained by the reaction of 2,6-dihydroxypyromellitimide [28] 14 with anisole in presence of anhydrous aluminum chloride (Scheme 6). The chemical structures of 10b and 11b were established on the basis of NMR spectrocopy, which were found to be completely fit with the proposed structures. 13C-NMR data were found to be ideal technique for the differentiation between the two isomers 10b and 11b. Compound 10b revealed the presence of ten different carbon signals, while 11b showed eleven different carbon signals, which were in good agreement with the proposed structures.
The reaction of 14 to give the two isomers 10b and 11b may proceed according to the mechanism shown in (Scheme 7).
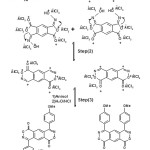 |
Scheme 7 Click here to View scheme |
Also, treatment of the acids 8a-d and/or 9a-d with hydrazine hydrate and/or phenylhydrazine gave the corresponding pyridazino[4,5-g]phthalazine-1,6-diones 12a-h and/or pyridazino[4,5-g]phthalazine-1,9-diones 13a-f respectively (Scheme 8).
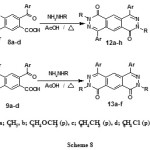 |
Scheme 8 |
The structure of compounds 12a-h and 13a-f were characterized by elemental analysis, IR and MS data.
1H-NMR spectra were not available because of low solubility of these compounds in organic solvents.
Conclusions
In summary, we have found that pyromellitic dianhydride is extremely useful for the synthesis of biologically relevant heterocyclic compounds such as dipyrroles, dibenzoxazines, dipyridazines and isophthalic and terephthalic derivatives in good yields after short reaction times.
Acknowledgement
Special thanks to Prof. Dr. T. Throll and Dr. Burgemeister (Regensburg University, Germany) for the IR help in the determenation of 13C-NMR, H-C COSY and elemental analysis.
Experimental
Melting points were uncorrected determined on an electric melting point apparatus (Kofler). The IR spectra (KBr) were recorded on a Shimadzu 408 spectrometer. The 1H-NMR spectra were recorded by 400 MHz Varian EM 390 spectrometer. The 13C-NMR spectra and The 1H-13C-NMR (H-C COSY) were measured on Avance 600 spectrometer; chemical shifts are reported in ppm with TMS as an internal standard and are given in δ units. Electron impact mass spectra were obtained at 70 ev using a GCMS sp.1000 Shimadzu. Elemental analyses were carried out at Microanalysis Unit at RegensburgUniversity.
Pyromellitic diimide 2.
A mixture of pyromellitic dianhydride 1 (1.09 g, 5 mmol) and the appropriate amide, namely, urea, thiourea and/ or thiosemicarbazide (20 mmol) was fused in an oil bath at 180o until the odour of ammonia was stopped (15 min). The residue was washed with water and crystallized from ethanol/DMF (2:1), as greenish white crystals in 93% yield; mp >3600;IR (KBr): ν 3250 cm-1 (NH) 1780 ; 1700 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 8.05 (s, 2H, two identical benzene protons) 11.82 (s, 2H, 2NH);; MS: m/z 216(M). Anal. Calcd. For C10 H4 N2 O4: C, 55.57; H, 1.87; N, 12.96; Found; C, 55.60; H, 1.77; N, 13.03%.
2,5-di[(benzylamino)carbonyl]terephthalic acid 3.
A mixture of pyromellitic dianhydride 1 (2.18 g, 10 mmol) and benzylamine (2.14 ml, 20 mmol) in toluene (50 ml) was refluxed for 1 hrs. After cooling, the solid crystals was filtered off and crystallized from acetic acid, as white crystals in 88% yield; mp 298-3000; IR (KBr): ν 3300 cm-1 (NH) 1710 ; 1660 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 4.43 (s, 4H, 2CH2), 7.22-7.43(m, 10H, arom.H), 7.77(s, 2H, two identical benzene protons), 9.17(s, 2H, 2COOH). Anal. Calcd. For C24H20N2O6: C, 66.66; H, 4.66; N, 6.48; Found; C, 66.71; H, 4.57; N, 6.52%.
2,6-dibenzylpyrrolo[3,4-f]isoindole-1,3,5,7(2H,6H)tetrone 4.
1.08 g (2.5 mmol) of 3 was fused at 310 o for 15 min. The solid formed was washed with water then ethanol and crystallized from DMF, as white crystals in 81% yield; mp 308-9o; IR (KBr): ν 1740 ; 1700 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 4.85 (s, 4H, 2CH2), 7.34 (m, 10H, arom.H), 8.22 (s, 2H, two identical benzene protons). Anal. Calcd. For C24H16N2O4: C, 72.72; H, 4.07; N, 7.07; Found; C, 72.69; H, 4.13; N, 7.04 %.
2,5-di(benz-1,3-thiazol-2-yl)terephthalic acid 5.
A mixture of pyromellitic dianhydride 1 (2.18 g, 10 mmol) and 2-aminothiophenol (2.5 g, 20 mmol) in glacial acetic acid (50 ml) was refluxed for 5 hrs. After cooling, the solid precipitated was filtered off and crystallized from DMF/ acetic acid (4:1), as white crystals in 83% yield; mp 335-37o; IR (KBr): ν 3600-3250 cm-1 (COOH), 1779, 1726 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.51-8.25 (m, 10H, arom.H), 13.8 (s, 2H, 2COOH); MS: m/z =432(M). Anal. Calcd. For C22 H12 N2O4S2: C, 61.10; H, 2.80; N, 6.48; S, 14.82; Found; C, 61.21; H, 2.84; N, 6.42; S, 14.73 %.
2,5-di(benz-1,3-thiazol-2-yl)benzoic acid 6.
Pyromellitic dianhydride 1 (2.18 g, 10 mmol) and 2-aminothiophenol (2.5 g, 20 mmol) in toluene (50 ml) were refluxed for 10 hrs. After cooling, the solid precipitated was filtered off and crystallized from ethanol, as green crystals in 77% yield; mp 323-250; IR (KBr): ν 3600-3250 cm-1 (COOH), 1707cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.36-8.25 (m, 11H, arom.H), 13.65 (s,1H, COOH); MS: m/z 388(M). Anal. Calcd. For C21 H12 N2O2S2: C, 64.92; H, 3.11; N, 7.21; S, 16.51; Found; C, 64.96; H, 3.18; N, 7.14; S, 16.47 %.
1,4-di(benz-1,3-thiazol-2-yl)benzene 7.
1 g (2.6 mmol) of 6 was fused at 320oC for 5 min. The solid formed was washed with water then sodium bicarbonate solution and crystallized from benzene, as brown crystals in 68% yield; mp 160-620; MS: m/z 344(M). Anal. Calcd. For C20 H12 N2S2: C, 69.74; H, 3.51; N, 8.13; S, 18.62; Found; C, 69.80; H, 3.47; N, 8.20; S, 18.53 %.
2,5-diaroylterephthalic acid 8a-d and 2,6-diaroylisophthalic acid 9a-d.
General procedure
Anhydrous aluminium chloride (8 g, 60 mmol) was added gradually while stirring to pyromellitic dianhydride (2.18 g, 10 mmol) in dry aromatic substrate, namely, benzene, anisole, toluene and/or chlorobenzene (30 ml). The reaction mixture was refluxed for 2 hrs. and then left overnight. The complex formed was decomposed with ice-cold dilute hydrochloric acid (1:1). The solvent was steam distilled, and after cooling, the solid isomers precipitated (83-94% yield) and were separated by column chromatography (30 cm height, 3 cm diameter) using benzene/ethanol (4:1) as an eluent to give 8a-d and 9a-d as white crystals.
2,5-dibenzoylterephthalic acid 8a.
Compound 8a was obtained in 56% yield, crystallized from ethanol/benzene (3:2); mp 320-220; IR (KBr) ν 3400-2550 cm-1 (COOH), 1750; 1680 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.52-7.75 (m, 10H, arom.H), 7.92 (s, 2H, two identical benzene protons) and disappearance of COOH protons. Anal. Calcd. For C22H14O6: C, 70.59; H, 3.77. Found; C, 70.56; H, 3.80%.
2,5-di(4-methoxybenzoyl)terephthalic acid 8b.
Compound 8b was obtained in 49% yield, crystallized from ethanol/benzene (2:1); mp 325-270; IR (KBr) ν 3200-2500cm-1 (COOH), 1700;1660 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ = 3.81 (s, 6H, 2 OCH3), 7.04-7.72 (dd, A2B2 system, 8H arom.), 7.85 (s, 2H, two identical benzene protons) and disappearance of COOH protons; MS: m/z 434. Anal. Calcd. For C24H18O8: C, 66.36; H, 4.18. Found; C, 66.40; H, 4.14 %.
2,5-di(4-methylbenzoyl)terephthalic acid 8c.
Compound 8c was obtained in 52% yield, crystallized from ethanol/benzene (3:2); mp 310-120; IR (KBr) ν 3400-2800 cm-1 (COOH), 1720;1680 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 2.37 (s, 6H, 2 CH3), 7.32-7.65 (dd, A2B2 system, 8H arom.), 7.88 (s, 2H, two identical benzene protons) and disappearance of COOH protons. Anal. Calcd. For C24H18O6: C, 71.64; H, 4.51. Found; C, 71.62; H, 4.52%.
2,5-di(4-chlorobenzoyl)terephthalic acid 8d.
Compound 8d was obtained in 55% yield, crystallized from ethanol/benzene (3:1); mp 320-230; IR (KBr) ν 3400-2800cm-1 (COOH), 1720;1680cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.55-7.79 (dd, A2B2 system, 8H arom.), 7.85 (s, 2H, two identical benzene protons) and disappearance of COOH protons; 13C-NMR (DMSO-d6): 128.82, d, C-2; 128.91, d, C-9; 130.90, d, C-3; 133.40 , s, C-4; 135.16, s, C-1; 138.40, s, C-6; 141.82, s, C-7; 165.63, s, C-5; 194.05, s, C-8. Anal. Calcd. For C22H12Cl2O6: C, 59.62; H, 2.73; Cl, 15.99. Found; C, 59.65; H, 2.74; Cl, 15.95 %.
4,6-dibenzoylisophthalic acid 9a.
Compound 9a was obtained in 38% yield, crystallized from ethanol/benzene (1:3); mp 278-790; IR (KBr) ν 3300-2800 cm-1 (COOH), 1720;1670 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.78-7.76 (m, 11H, arom.H + H-5), δ 8.85 (s, 1H, H-2) and disappearance of COOH signal. Anal. Calcd. For C22H14O6: C, 70.59; H, 3.77. Found; C, 70.61; H, 3.75 %.
4,6-di(4-methoxybenzoyl)isophthalic acid 9b.
Compound 9b was obtained in a 34% yield, crystallized from ethanol/benzene (1:3); mp 215-170; IR (KBr) ν 3300-2500 cm-1 (COOH), 1730; 1680 cm-1 (C=O’s); 1H-NMR (CDCl3): δ 33.85 (s, 6H, 2OCH3), 7.10-7.65 (dd, A2B2 system, 8H arom.), 7.40 (s, 1H, H-5), ) 8.55 (s, 1H, H-2), 13.85 (s, 2H, 2COOH); 13C-NMR (CDCl3): 55.59, q; 114.04, d; 126.41, d; 129.29, s; 130.50, s; 131.39, d; 131.44, d; 144.95, s; 163.36, s; 165.63, s; 193.56, s.; MS: m/z 434 (M). Anal. Calcd. For C24H18O8: C, 66.36; H, 4.18. Found; C, 66.39; H, 4.15 %.
4,6-di(4-methylbenzoyl)isophthalic acid 9c.
Compound 9c was obtained in 40% yield, crystallized from ethanol/benzene (2:3); mp 248-500; IR (KBr) ν 3250-2500 cm-1 (COOH), 1700,1670 cm-1 (C=O’s); 1H-NMR (CDCl3): δ 2.40 (s, 6H, 2CH3), 7.22-7.37 (dd, A2B2 system, 8H arom.), 7.31 (s, 1H, H-5), 8.77 (s, 1H, H-2), 9.49 (s, 2H, 2COOH); MS: m/z 402(M). Anal. Calcd. For C24H18O6. C, 71.64; H, 4.51. Found; C, 71.61; H, 4.53%.
4,6-di(4-chlorobenzoyl)isophthalic acid 9d.
Compound 9d was obtained in 33% yield, recrystallized from ethanol/benzene (1:2); mp 240-420; IR (KBr) ν 3400-2270 cm-1 (COOH), 1740,1700 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.56-7.72 (dd, A2B2 system, 8H arom.), 7.95 (s, 1H, H-5), 8.55 (s, 1H, H-2) and disappearance of COOH protons; 13C-NMR (DMSO-d6): 128.84, d, C-2, C-9; 130.67, d, C-3; 131.38, d, C-10; 135.03, s, C-1; 138.30, s, C-4; 141.73, s, C-6; 144.41, s, C-7; 165.56, s, C-5; 193.91, s, C-8; MS: m/z 442(M). Anal. Calcd. For C22H12Cl2O6: C, 59.62; H, 2.73; Cl, 15.99. Found; C, 59.66; H, 2.74; Cl, 15.94%.
Synthesis of diaryl-[1,2]oxazinobenzoxazines 10a-d and 11a-d.
General procedure
To a solution of 2,5-diaroylterephthalic acid 8a-d and/or 4,6-diaroylisophthalic acid 9a-d (5 mmol) in dry pyridine (10 ml), hydroxylamine hydrochloride (1.38 g, 20 mmol) was added, then the reaction mixture was refluxed for 1 hr. After cooling, the reaction mixture was poured onto ice-dilute hydrochloric acid, the solid precipitated was filtered off and crystallized from appropriate solvent to give 10a-d and/or 11a-d white crystals.
4,9-diphenyl-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione 10a.
Compound 10a was obtained in 60% yield, crystallized from Ethanol/DMF (6:1); mp >3600; IR (KBr) ν 1740 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.69(m, 10H , arom. H), 8.15 (s, 2H, two identical benzene protons). Anal. Calcd. For C22H12N2O4: C, 71.74; H, 3.28; N, 7.61. Found; C, 71.71; H, 3.27; N, 7.65 %.
4,9-di(4-methoxyphenyl)-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione10b.
Compound 10b was obtained in 57% yield, recrystallized from acetic acid; mp 355-580; IR (KBr) ν 1750 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 3.72 (s, 6H, 2OCH3), 6.89-7.13 (dd, A2B2 system, 8 H arom.), 7.72 (s, 2H, two identical benzene protons); MS: m/z 428(M). Anal. Calcd. For C24H16N2O6: C, 67.29; H, 3.76; N, 6.54. Found; C, 67.32; H, 3.71; N, 6.56 %.
4,9-di(4-methylphenyl)-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione 10c.
Compound 10c was obtained in 66% yield, crystallized from DMF; mp 350-520; IR (KBr) ν 1750 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 2.45 (s, 6H, 2CH3), 7.48-7.57 (dd, A2B2 system, 8 H arom.), 8.17 (s, 2H, two identical benzene protons); MS: m/z 396(M). Anal. Calcd. For C24H16N2O4: C, 72.72; H, 4.07; N, 7.07. Found; C, 72.77; H, 4.05; N, 7.04%.
4,9-di(4-chlorophenyl)-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione 10d.
Compound 10d was obtained in 70% yield, crystallized from methanol; mp 358-590; IR (KBr) ν 1750; 1740 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.71-7.79 (dd, A2B2 system, 8 H arom.), 8.13 (s, 2H, two identical benzene protons); MS: m/z 436(M). Anal. Calcd. For C22H10Cl2N2O: C, 60.43; H, 2.31; N, 6.41; Cl, 16.22. Found; C, 60.39; H, 2.35; N, 6.46; Cl, 16.16%.
4,6-diphenyl-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione 11a.
Compound 11a was obtained in 56% yield, crystallized from DMF; mp 305-80; IR (KBr): ν 1760 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.52-7.69(m, 11H, arom. H + H-5), 8.96 (s, 1H, H-10). Anal. Calcd. For C22H12N2O4: C, 71.74; H, 3.28; N, 7.61. Found; C, 71.78; H, 3.30; N, 7.55%.
4,6-di(4-methoxyphenyl)-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione11b.
Compound 11b was obtained in 69% yield, crystallized from chloroform, mp 278-790; IR (KBr) ν = 2932-2832 cm-1 (CH3), 1703 cm-1 (C=O’s); 1H-NMR (DMSO): δ 3.72 (s, 6H, 2OCH3), 6.83-7.10 (dd, A2B2 system, 8 H arom.), 7.62 (s, 1H, H-5), 7.97 (s, 1H, H-10). Anal. Calcd. For C24H16N2O6: C, 67.29; H, 3.76; N, 6.54. Found; C, 67.30; H, 3.74; N, 6.55%.
4,6-di(4-methylphenyl)-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione 11c.
Compound 11c was obtained in 72% yield, crystallized from DMF; mp 292-94 0C; IR (KBr) ν 1755 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 2.39 (s, 6H, 2CH3), 7.39-7.48 (dd, A2B2 system, 9 H, arom. H+ H-5), 8.97 (s, 1H, H-10). Anal. Calcd. For C24H16N2O4: C, 72.72; H, 4.07; N, 7.07. Found; C, 72.75; H, 4.09; N, 7.02%.
4,6-di(4-chlorophenyl)-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione 11d.
Compound 11d was obtained in 76% yield, crystallized from acetic acid; mp 310-120; IR (KBr) ν 1760, 1730 cm-1 (C=O’s); 1H-NMR (CDCl3): δ 7.24-7.50 (dd, A2B2 system, 9 H arom. H+ H-5) , 7.62 (s, 1H, H-10). Anal. Calcd. For C22H10Cl2N2O: C, 60.43; H, 2.31; N, 6.41; Cl, 16.22. Found; C, 60.46; H, 2.33; N, 6.40; Cl, 16.17%.
4,9-di(4-methoxyphenyl)-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione 10b and 4,6-di(4-methoxyphenyl)-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione 11b.
Anhydrous aluminum chloride (8 g, 60 mmol) was added gradually while stirring to 2,6-dihydroxypyrrolo[3,4-f]isoindolo-1,3,5,7(2H,6H)tetrone 14 (2.48g, 10 mmol) in dry anisole (20 ml). The reaction mixture was stirred for 1.5 hrs. left overnight and the complex formed was decomposed with ice-cold dilute hydrochloric acid (1:1). The solvent was steam distilled, the crude solid formed was filtered off and the two isomers were separated using hot ethanol. The insoluble isomer was filtered off and crystallized from acetic acid to give 10b. The soluble isomer was isolated and crystallized from chloroform to give 11b.
4,9-di(4-methoxyphenyl)-1H,6H[1,2]oxazino[5,4-g][2,3]benzoxazine-1,6-dione10b.
Compound 10b was obtained in 45% yield; mp 355-580; IR (KBr) ν 1690 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 3.75 (s, 6H, 2OCH3), 6.85-7.13 (dd, A2B2 system, 8H, arom. H), 7.72 (s, 2H, two identical benzene protons); 13C-NMR (DMSO-d6): 55.08, q, C-1; 158.95, s, C-2; 113.76, d, C-3; 129.27, d, C-4; 74.00, s, C-5; 146.98, s, C-6; 161.03, s, C-7; 132.29, s, C-8; 131.56, s, C-9; 118.43, d, C-10. Anal. Calcd. For C24H16N2O6: C, 67.29; H, 3.76; N, 6.54. Found; C, 67.33; H, 3.74; N, 6.52%.
4,6-di(4-methoxyphenyl)-1H,9H[1,2]oxazino[4,5-g][2,3]benzoxazine-1,9-dione11b.
Compound 11b was obtained in 25% yield; mp 278-890; IR (KBr) ν 1680 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 3.73 (s, 6H, 2OCH3), 6.87-7.08 (dd, A2B2 System, 8H, arom. H) and two non identical protons at 7.59 (s, 1H, H-5) and at 7.95 (s, 1H, H-10); 13C-NMR (DMSO-d6): 55.12, q, C-1; 158.89, s, C-2; 113.60, d, C-3; 129.30, d, C-4; 74.02, s, C-5; 150.68, s, C-6; 161.01, s, C-7; 131.55, s, C-8, C-9 (Interfered); 117.06, d, C-10; 120.12, d, C-11. Anal. Calcd. For C24H16N2O6: C, 67.29; H, 3.76; N, 6.54. Found; C, 67.32; H, 3.75; N, 6.52%.
Synthesis of pyridazinophthalazines 12a-h and 13a-f.
General procedure
Hydrazine hydrate (20 mmol) and/or phenylhydrazine (10 mmol) was added to a solution of 2,5-diaroylterephthalic acid 8a-d and/or 4,6-diaroylisophthalic acid 9a-d in acetic acid (30 ml). The reaction mixture was refluxed for 2 hrs and after cooling the solid formed was filtered and washed with DMF to give 12a-h as white crystals and/or 13a-f as yellow crystals respectively.
4,9-diphenyl-pyridazino[4,5-g]phthalazine-1,6(2H,7H)dione 12a.
Compound 12a was obtained in 78% yield; mp >3600; IR (KBr) ν 3150 cm-1 (NH) 1660 cm-1 (C=O’s); MS: m/z 366(M). Anal. Calcd. For C22H14N4O2: C, 72.12; H, 3.85; N, 15.29. Found; C, 72.18; H, 3.81; N, 15.28%.
4,9-di(4-methoxyphenyl)-pyridazino[4,5-g]phthalazine-1,6(2H,7H)dione 12b.
Compound 12b was obtained in 81% yield; mp >3600; IR (KBr) ν 3200 cm-1 (NH), 1665cm-1 (C=O’s); MS: m/z 426(M). Anal. Calcd. For C24H18N4O4: C, 67.60; H, 4.25; N, 13.14. Found; C, 67.58; H, 2.29; N, 13.12%.
4,9-di(4-methylphenyl)-pyridazino[4,5-g]phthalazine-1,6(2H,7H)dione 12c.
Compound 12c was obtained in 73% yield; mp >360 0C; IR (KBr) ν 3200 cm-1 (NH), 1660cm-1 (C=O’s); MS: m/z 394(M). Anal. Calcd. For C24H18N4O2: C, 73.08; H, 4.60; N, 14.20. Found; C, 73.11; H, 4.65; N, 14.13%.
4,9-di(4-chlorophenyl)-pyridazino[4,5-g]phthalazine-1,6(2H,7H)dione 12d.
Compound 12d was obtained in 84% yield; mp >3600; IR (KBr) ν 3200 cm-1 (NH), 1680cm-1 (C=O’s); MS: m/z 434(M). Anal. Calcd. For C22H12Cl2N4O2: C, 60.71; H, 2.78; N, 12.87; Cl, 16.29. Found; C, 60.69; H, 2.81; N, 12.85; Cl, 16.30%.
4,9-diphenyl-2,7-diphenylpyridazino[4,5-g]phthalazine-1,6-dione 12e.
Compound 12e was obtained in 83% yield, crystallized from xylene; mp >360 0C; IR (KBr) ν 1670 cm-1 (C=O’s); MS: m/z 518(M). Anal. Calcd. For C34H22N4O2: C, 78.75; H, 4.28; N, 10.80. Found; C, 78.79; H, 4.30; N, 10.74%.
4,9-di(4-methoxyphenyl)-2,7-diphenylpyridazino[4,5-g]phthalazine-1,6-dione 12f.
Compound 12f was obtained in 85% yield, crystallized from xylene; mp 357-590; IR (KBr) ν 1670 cm-1 (C=O’s); MS: m/z 578(M). Anal. Calcd. For C36H26N4O4: C, 74.73; H, 4.53; N, 9.68. Found; C, 74.78; H, 4.57; N, 9.59%.
4,9-di(4-methylphenyl)-2,7-diphenylpyridazino[4,5-g]phthalazine-1,6-dione 12g.
Compound 12g was obtained in 81% yield, crystallized from xylene; mp >3600; IR (KBr) ν 1670 cm-1 (C=O’s); MS: m/z 546(M). Anal. Calcd. For C36H26N4O2: C, 79.10; H, 4.79; N, 10.25. Found; C, 79.16; H, 4.81; N, 10.18%.
4,9-di(4-chlorophenyl)-2,7-diphenylpyridazino[4,5-g]phthalazine-1,6-dione 12h.
Compound 12h was obtained in 88% yield, crystallized from DMF; mp >3600; IR (KBr) ν 1680 cm-1 (C=O’s); MS: m/z 586(M). Anal. Calcd. For C34H20Cl2N4O2: C, 69.51; H, 3.43; N, 9.54; Cl, 12.07. Found; C, 69.50; H, 3.45; N, 9.56; Cl, 12.04%.
4,6-diphenyl-pyridazino[4,5-g]phthalazine-1,9(2H,8H)dione 13a.
Compound 13a was obtained in 74% yield; mp >3600; IR (KBr) ν 3300-3200 cm-1 (NH), 1700 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.47-7.63(m, 10H, arom. H ), 8.02 (S, 1H, H-5), 9.07 (s, 1H, H-10), 13.10 (s, 2H, 2NH). Anal. Calcd. For C22H14N4O2: C, 72.12; H, 3.85; N, 15.29. Found; C, 72.15; H, 3.88; N, 15.24%.
4,6-di(4-methoxyphenyl)-pyridazino[4,5-g]phthalazine-1,9(2H,8H)dione 13b.
Compound 13b was obtained in 75% yield; mp >3600; IR (KBr) ν 3494; 3447 cm-1 (NH), 1688 cm-1 (C=O’s); MS: m/z 426(M). Anal. Calcd. For C24H18N4O4: C, 67.60; H, 4.25; N, 13.14. Found; C, 67.62; H, 4.27; N, 13.10%.
4,6-di(4-methylphenyl)-pyridazino[4,5-g]phthalazine-1,9(2H,8H)dione 13c.
Compound 13c was obtained in 82% yield; mp >360 0C; IR (KBr) ν 3200 cm-1 (NH), 1660 cm-1 (C=O’s); MS: m/z 394(M). Anal. Calcd. For C24H18N4O2: C, 73.08; H, 4.60; N, 14.20. Found; C, 73.06; H, 4.64; N, 14.18%.
4,6-di(4-chlorophenyl)-pyridazino[4,5-g]phthalazine-1,9(2H,8H)dione 13d.
Compound 13d was obtained in 72% yield; mp >3600; IR (KBr) ν 3200 cm-1 (NH), 1680cm-1 (C=O’s); MS: m/z 434(M). Anal. Calcd. For C22H12Cl2N4O2: C, 60.71; H, 2.78; N, 12.87; Cl, 16.29. Found; C, 60.76; H, 2.72; N, 12.82; Cl, 16.35%.
2,4,6,8-tetraphenyl-pyridazino[4,5-g]phthalazine-1,9-dione 13e.
Compound 13e was obtained in 78% yield, crystallized from xylene; mp 324-260. IR (KBr) ν 1680 cm-1 (C=O’s); MS: m/z 518 (M). Anal. Calcd. For C34H22N4O2: C, 78.75; H, 4.28; N, 10.80. Found; C, 78.79; H, 4.25; N, 10.79%.
4,6-di(4-chlorophenyl)-2,8-diphenyl pyridazino[4,5-g]phthalazine-1,9-dione 13f.
Compound 13f was obtained in 64% yield, crystallized from Xylene / DMF; mp= 3600; IR (KBr) ν 1680 cm-1 (C=O’s); 1H-NMR (DMSO-d6): δ 7.53-7.81 (m, 19H, 18 arom. H + H-5) 8.67 (s, 1H, H-10); MS: m/z 586 (M). Anal. Calcd. For C34H20Cl2N4O2: C, 69.51; H, 3.43; N, 9.54; Cl, 12.07. Found; C, 69.53; H, 3.45; N, 9.56; Cl, 12.01%.
References
- Choi, J. K.; Yoon, T. H., Journal of Applied Polymer Science, 2(117), 736–741 (2010).
- , M.; Ghaemy, M.; Hassanzadeh, M., High Performance Polymers, 4(24),4 305-318 (2012).
- Jain, R.; Choudhary, V.; Narula, A. K., Journal of Applied Polymer Science, 4(106), 2593–2598, (2007).
- Bo Tao; Hua Xia; Chao-Xin, H.; Xiao-Wei Li, Zeitschrift für anorganische und allgemeine Chemie, 6 (637), 703–707
- Tellez, H., M.; Alquisira, J. P.; López-Cortés, J. G.; Alvarez-Toledano, C., Journal of Applied Polymer Science, 5(116), 2816–2824 (2010).(2011).
- Baruah, A. M.; Karmakar, A.; Baruah, J. B., Polyhedron, 15(26), 4479–4488 (2007).
- Bakhite, E. A.; Radwan, S. M.; K. El-Deen, A.M., J. Chin. Chem. Soc., 47(5), 1105 (2000).
- Younes, M. I.; Abbas, H. H.; Metwally, S. M., pharmazie, 46 (2), 98 (1991).
- Gatta, F.; Perotti, F.; Gradoni, L.; Gramiccia, M.; Orsini, S.; Palazzo, G.; Rossi, V., Eur. J. Med. Chem., (25), 419 (1990).
- Marei, M. G.; Aly, D. M.; Mishrikey, M. M., Bull. Chem. Soc., Jpn., 65 (12), 3419 (1992).
- Ugarkar, B. G.; Cottam, H. B.; Mckernan, P. A.; Robins, R.K.; Revankar, G.R., J. Med. Chem., 27 (8), 1026 (1984).
- Mishra, B.; Muddin, N., Indian J. Chem., (28B), 346 (1989).
- Miller, D. J.; Shen, H.; Suh, J. K.; Kerwin, S.M.; Robertuss, J. D., J. Med. Chem., 45 (1), 90 (2002).
- Shalaby, A. M.; Fathalla, O. A.; Kassem, E. M. M; Zaki, M. E. A., Acta Chim. Slov., (47), 187 (2002).
- El-Afaleq, E. I.; Abubshit, S. A., Molecules, (6), 621 (2001).
- Bhuyan, P. J.; Borah, H. N.; Lekhok, K. C.; Sanhu, J. S., J. Heterocyclic Chem., (38), 491 (2001).
- Finch R. A.; Revankar G. R.; Chan P. K., Anticancer Drug Desing, (12), 205 (1996).
- Newman, M. S.; Muth, C. W., J. Am. Chem. Soc., (73), 4657 (1951).
- Ostrowski, S.; Swat, J.; Makosza, M., Arkivoc, 1 (6), 905 (2000).
- Jain, R.; Shukla, A., J. Indian Chem. Soc., (67), 575 (1990).
- Hassan M. A.; Fahmy, A. F. M., Arch-pharm. (Weinheim), (321), 943-944 (1988).
- Fahmy, A. F. M.; Sauer J.; Yousef, M. S. K.; Abd El-Halim, M. S.; Hassan, M. A., J. Heterocycles, (24), 2201 (1986).
- Hassan, M. A.; Zayed, S. E.; El-GazIRi, W. N.; Saoud A. M., Bull. Fac. Sci., Assiut Univ., 19 (2-B), PP 75-85 (1990).
- Zayed, E. S.; Wafaa N.; Saoud A. M., Bull. Fac. Sci. Assuit Univ., 21(1-B), PP. 95-102 (1992).
- Ganin, E. V.; Makarov, V.F.; Nikitin, V. I., Zh. Org. Khim., 23(5), 1086-9 (1987).
- Literature Review, Chapter II, Synthesis of Polyimides (2006),(http://www.scolar.lib.vt.edu/theses/available/etd-080999-123034/unrestricted/07chapter2.pdf).
- Hotbson, W.; Mills, M., J. Chem. Soc, (101), 2191-2201 (1912).
- Dao, B.; Mortan, T., Ger. Offen. DE.,19, 540, 107 (Cl. CO7D 487/04), (1996); C. A., 125 (10), 115415f (1996).

This work is licensed under a Creative Commons Attribution 4.0 International License.









