Synthesis, Characterization and Properties of Metal Complexes of Beta-diketonate Complexes
Mukesh Kumar and T.R. Sharma
Department of Chemistry, K.G.K. College, Moradabad - 243 005, India.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
???????-diketone with oligo (ethylene oxide) group and its transition metal complexes were synthesized and characterized by ‘HNMR, IR, Electronic spectra, m.p., elemental analyses and magnetic studies. Based on these studies probable structures have been proposed for these metal chelates. .
KEYWORDS:HNMR; IR; β-diketone; and chelates
Download this article as:| Copy the following to cite this article: Kumar M, Sharma T. R. Synthesis, Characterization and Properties of Metal Complexes of Beta-diketonate Complexes. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Kumar M, Sharma T. R. Synthesis, Characterization and Properties of Metal Complexes of Beta-diketonate Complexes. Available from: http://www.orientjchem.org/?p=22783 |
Introduction
Transition metals play a central role in the construction of molecular materials, which display magnetic properties and find applications in material and supramolecular chemistry and biochemistry (1-4). Their complexes are well known for their biological importance as well as their anticarcinogenic, antibacterial and anti-fungal properties (5).
Bifunctional ligands based acetylacetonates are attractive for use in construction of mixed – metal networks. The chelating nature of bidentate O, O-donor ensures relatively low lability and the negative charge on the ligand allows access to neutral complexes. Keeping these facts in view, the present work involving the synthesis of the ligand containing b-diketone with oligo (ethylene-oxide) group and its transition metal complexes.
Experimental
The reagents and solvents were commercially available and of analytical purely. Dimethoxy ethane was strictly dried by heating under reflux with sodium sand, using benzophenone as indicator. The synthetic route to the present b diketone ligand and the corresponding transition metal complexes is shown in the scheme.
Synthesis Of The Ligand
The ligand 1-(p-ethoxy-di (ethyleneoxy) phenyl-3-(p-n-hexoxyl phenyl) propane-1,3-dione was synthesized by dissolving the mixture of 3.00g (1.19×10-2 ‑mol) of p-ethoxy-di (ethyleneoxy) acetophenone and 2.80g (1.18 x 10-2 mol) of methyl p-n hexyloxybenzoato in dimetho-xyethane and heating under reflux for 3h in presence of 0.948g of 60% sodium hydride (2.37 x 10-2 mol). The resulting brown- yellow solution was cooled to room temperature and stirred overnight. Small portions of ethanol and then water were added carefully to the resulting orange mixture. After acidifying the mixture with dilute hydrochloric acid, the product was extracted into ether. Crude ligand was obtained by evaporating the solvent. This was purified by chromatography on silica eluted with a mixed solvent of hexane and ethyl acetate in the volume ratio of 2:1 to pale yellow crystals of the ligand.
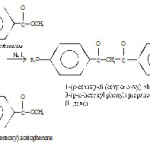 |
Scheme 1 |
Preparation Of Metal Complexes
A mixture of the ligand and respective metal salt and 50 ml of anhydrous ethanol was stirred for 3h at room temperature. Solid product was precipitated in each case, which was washed with ethanol and recrystalised from ethyl acetate. The metals used for this purpose were TiCl3, FeCl3, VCl3. MnCl3, CoCl3, RuCl3, RuCl2.
Characterization Of Ligand & Metal Complexes
The b-diketone and its transition metal complexes were subjected to elemental analyses while metals were estimated gravimetrically in the lab. Their melting points were determined by open capillary method and are uncorrected. These were characterized by molar conductance, magnetic susceptibility measurement, I.R., electronic ‘H NMR spectra and TGA.
Result And Discussion
The analytical data suggested 1:2 M:L stiochiometry for all the synthesized complexes. The molar conductance values determined at 10-3M dilution and 250C suggested 1:1 electrolytic nature for M (III) complexes and non-electrolytic nature for M (II) complex. The spin only values of the complexes suggested paramagnetic nature for Ti (III), V (III), Mn (III), Ru (III) and Fe (III) complexes and diamagnetic nature for Co (III) and Ru (II) complexes.
Table-1: Analytical Data of the Ligand and Metal Complexes
| Sl No | Mol. for of ligand/ Complex | Colour | M.P. 0C | Elemental analyses | Magnetic Moment (B.M.) | Molar Conductance in DMF (ohm-1 cm2 mole-1) | ||
| % of C | % of H | %of M | ||||||
| 1. | LigandC27H36O6 Mol. Wt.=456 | Pale Yellow | 154 | 69.54 (71.02) | 7.78 (7.94) | – | – | – |
| 2. | [Ti(C27H35O6)2.2H2O] ClMol. Wt.= 1065 | Deep Yellow | 198 | 59.76 (60.67) | 6.82 (6.94) | 4.31 (4.50) | 1.68 | 65 (1:1 electrolyte) |
| 3. | [V(C27H35O6)2.2H2O] ClMol. Wt. = 1068 | Yellow | 199 | 59.82 (60.67) | 6.84 (6.92) | 4.69 (4.77) | 2.92 | 60 (1:1 electrolyte) |
| 4. | [Mn(C27H35O6)2.2H2O] ClMol.Wt. = 1072 | Dark Brown | 201 | 59.10 (60.44) | 6.80 (6.90) | 4.89 (5.13) | 4.93 | 55 (1:1 electrolyte) |
| 5. | [Ru(C27H35O6)2.2H2O] ClMol. Wt. = 1118 | Green | 232 | 56.88 (57.96) | 5.96 (6.61) | 8.71 (9.03) | 1.98 | 68 (1:1 electrolyte) |
| 6. | [Fe(C27H35O6)2.2H2O] ClMol. Wt. = 1073 | Brown | 203 | 59.72 (60.39) | 6.03 (6.89) | 4.88 (5.21) | 5.87 | 72 (1:1 electrolyte) |
| 7. | [Ru(C27H35O6)2.2H2O]Mol. Wt. = 1083 | White | 228 | 58.20 (59.83) | 6.76 (6.83) | 8.66 (9.32) | Diamagnetic | Non – electrolyte |
| 8. | [Co(C27H35O6)2.2H2O] ClMol. Wt. = 1076 | Black | 232 | 59.46 (60.22) | 6.79 (6.87) | 4.91 (5.48) | Diamagnetic | 65 (1:1 electrolyte) |
Calculated values are given in brackets
Infra Red & ‘H Nmr Spectra
Both these spectral studies showed the ligand is in the state of enolic form.
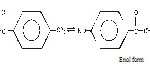 |
Scheme 2 |
The comparison of the i.r and H’ NMR spectra of the ligand and its respective metal complexes suggested monobasic bidentate (O, O’) nature of the ligand, since the frequencies of these groups undergo shifts on complexation. The first coordination site is nC=O group as its band at 1675 cm-1 shifts and found in the range of 1682-1687 cm-1 in the complexes. The other coordination site is the depronated nC-O group as ligand band due to nOH disappears and a new band appears in the i.r. spectra of the complexes in the range of 1275-1260 cm-1 assignable to phenolic nC-O obsorption (6). This indicated that deprotonation accurs prior to the coordination. This is further supported by a new band in the complexes in the region of 520-540 cm-1 due to nM-O frequency (7). The i.r. spectra of the complexes showed non-ligand bands in the region of 3490-3510 cm-1 due to nO-H, which indicated the coordinated nature of water molecules. This was further supported by two other bands in the regions of 840-850 cm-1 and 735-755 cm-1 assignable to rocking and wagging modes of coordinated water molecules (8). TGA also supported this inference of IR spectra regarding coordinated nature of water molecules. The thermograms showed the inflection points corresponding to the loss of two water molecules in each case between the temperature range of 1650Cto 1900C.
Electronic Spectra
The electronic spectrum of Ti complex shows a single broad band at 19560 cm-1 due to 2At2g → 2Eg transition for Oh symmetry (9). The value of magnetic moment of this complex is 1.68 B.M. which is in the expected range for d1 system like Ti (III) and shows paramagnetic character of the complex. It also shows that Ti (III) has not been oxidized to Ti (IV) during or after complexation, although it is very sensitive to oxidation.
The Mn (III) complex shows a magnetic moment of 4.93 B.M., corresponding to the presence of four unpaired electrons and high spin state of the complex. This value also suggests the absence of any kind of exchange interaction (10). The electronic spectrum of complex shows an intense and sharp charge transfer band at 22000 cm-1 and a spin allowed d-d transition at 18540 cm-1 characteristic of octahedral geometry.
The study of magnetic properties of the Co (III) complex indicated diamagnetic nature, as expected for a low spin d6 ion. The electronic spectrum of the chelate displays bands at 15230. 21195 and 23410 cm-1 assignable to , and transitions respectively. These are characteristic of low spin octahedral complexes of Co (III) (11).
The observed value of effective magnetic moment of the complex is 2.92 B.M., as expected for d2 system like V (III). The electronic spectrum of the complex exhibits a band at 16110 cm-1 with a shoulder at 20530 cm-1. The low energy band may be assigned to and the high energy band to . These are characterises of octahedral geometry(12).
The Ru (III) complex shows a magnetic moment of 1.98 B.M. and three bands in its electronic spectrum at 13650. 17600 and 22500 cm-1 have been observed. These bands are assigned to . and,respectively, and are similar to those reported for other Ru (III) octahedral complexes (13).
The Ru (II) complex is diamagnetic in nature which shows +2 oxidation state for Ru (II) in this complex. The electronic spectrum of the complex in CH2Cl2 shows a band assigned to the charge transfer transition arising from the excitation of an electron from the metal t2g level to the unfilled molecular orbitals derived from the p* level of the ligand in accordance with the assignments made for other similar octahedral Ru (II) complex (14).
The magnetic moment of Fe (III) complex is 5.87 B.M. corresponding to the presence of five unpaired electrons and high spin state of Fe (III) in this complex. The electronic spectrum of this complex shows three bands at 12720, 19600 and 25000 cm-1 due to (G), (G) and (G) transition respectively in an octahedral symmetry(15).
Conclusion
Based on the studies performed and discussed above, octahedral geometry has been proposed for all the synthesized complexes.
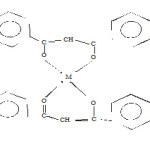 |
Scheme 3 |
R1 = CH3 CH2 OCH2 CH2 OCH2 CH2
R2 = C6 H13
Where M = Ti(III), Fe(III), V (III), Mn (III),
Co (III) or Ru (III), X=Cl
Where M = Ru (II), X = 0
References
- S. Chandra and R. Kumar, Trans. Met. Chem., 29, 269, (2004).
- S. Chandra and R. Kumar, Synth. and React. In Inorg. Met. Org. and Nano-Metal Chem. 35, 103 (2005).
- S. Chandra and R. Kumar, Synth. and React. In Inorg. Met. Org. and Nano-Metal Chem. 35, 161 (2005).
- E. Madej, O. Monsted and P. Kita, J. Chem. Soc. Dalton Trans., 2361 (2002)
- A. Levana, R. Codd, C.T. Dillon and P.A. Lay, Prog. Inorg. Chem. 51, 145 (2002).
- T. Daniel Thangadurai & K. Natrajan, Indian Journal of Chemistry, 41A, pp. 741-745 (2002).
- Ramvir, Neeti Gupta & Nighat Fahmi, Indian Journal of Chemistry, 38A, pp. 1150-1158 (1999).
- B. Rupini, K. Mamatha, R. Mogili, M. Ravinder and S. Srihari J. Indian Chem. Soc., 84, pp. 629-631 (2007).
- Samik K Gupta, Roy S, Mandal TN, Das K., J. Chem. Sci. 122 (2010) 239.
- Rahul K Rastogi, Poonam Garg & Shamim Ahmad, Asian J. Chem. 21 (2009) 6144.
- T. Daniel Thangaduvai & K. Natrajan, Indian J. Chem. 41(A) (2002) 741.
- Kamalendu Dey, Bhattacharya PK, Bandyo Padhyay & Chakraborty K, Indian J. Chem. 35(A) (1996) 580.
- Mahesh K. Singh, Singh AK, Gupta PK, Jaipal & Sharma LK, Indian J. Chem. 41 (2002) 1385.
- Natrajan K, Poddar RK and Agrawal U, J Inorg. Nucl. Chem. 39 (1977) 431.
- Liver AB, Inorganic electronic spectroscopy and Adn. (Exevier, Amsterdam) 1984 and references therein.

This work is licensed under a Creative Commons Attribution 4.0 International License.









