Synthesis and Spectral Studies of Some Transition metal Complexes
Daya Veer and Mukesh Baboo
Department of Chemistry, Hindu College, Moradabad - 244 001, India.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Complexes of M(III) & M(II) transition metals with N, N’-ethylene-bis-(2-amino-benzamide) have been prepared and characterized by elemental analyses, molar condcutivity, magnetic susceptibility measurments, thermal studies , IR and electronic spectra. Based on these studies octahedral geometry has been proposed for these complexes.
KEYWORDS:Schiff base; Octahedral; Transition Metal
Download this article as:| Copy the following to cite this article: Veer D, Baboo M. Synthesis and Spectral Studies of Some Transition metal Complexes. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Veer D, Baboo M. Synthesis and Spectral Studies of Some Transition metal Complexes. Available from: http://www.orientjchem.org/?p=22765 |
Introduction
Schiff bases are an important class of ligands in coordination chemistry and their complexing ability containing different donor atoms is widely reported.1,2 The chemistry of Transition metal complexes containing schiff bases continues to be of interest on account of their interesting structural features and also because of their biological importance.3,4
The coordination complexes of the schiff bases have been widely investigated due to their manifestation of novel structural features, unusual magnetic properties and relevance to biological process. 5-7
The polydentate schiff bases are well known to coordinate with various metal ions to form metallic complexes with theoretical and practical applications of different type.8 These ligand systems have gained considerable importance because of their utility as model compounds in bioinorganic studies as well as their potential as catalysts in many reactions9. They find application in the determination of transition metal ions10,11,12 in the study of acid base equilibrium acidity constants value13 & for analytical purposes14.
Experimental
All the chemicals and reagents used for this research work were of highest purity or A.R. Grade. The chemicals used were 2-aminobenzamide and ethylene dichloride. The solvents used were methanol, ethanol and DMF. Ethanol was double distilled in the lab before use whereas other solvents were used as procured. The metals used were Fe (III), Ti(III), Mn(III), V(III), Co(III), Ru(III), MoO(V), MoO2(VI) UO2(VI) & Ru(II). Ti(III) chloride was prepared in the lab by standard method while all other metal salts were used as procured.
Preparation Of Ligand
Preparation of N,N’-ethylene-bis-(2-aminobenzamide)
1 mole of ethylene dichloride and 2 mole of 2-aminobenzamide were dissolved in methanol & refluxed for 3 hr. The solid product so obained was filtered, washed with methanol and dried in vacuum dessicator.
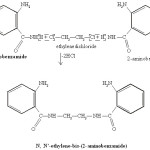 |
Scheme 1 Click here to View Scheme |
Preparation Of Complexes
N, N’-ethylene-bis-(2-aminobenzamide) was dissolved in methanol, the pH of the solution was adjusted to 6.O by adding methanolic solution of NaOH. The metal salt was dissolved in methanol and gradually added to the solution of the ligand with constant stirring. The microcrystalline complexes formed were suction filtered and washed with hot methanol several times. The products were dried in vacuo over anhydrous calcium chloride.
Characterization Of Ligand And Metal Complexes
The colour of the ligand and its metal complexes were noted. The m.p. of the ligand and its complexes were determined by open capillary method and are uncorrected. The ligand and its complexes were subjected to elemental analyses for C, H and N whereas metal was estimated gravimetrically in the Lab. The electronic spectra of the complexes were recorded with the help of Beckman DU. The I.R. spectra were recorded at CDRI, Lucknow in KBr phase.
Results & Discussion
The complexes are stable in air except those of Ti(III) & V (III) and are non hygroscopic. These are insoluble in water and common organic solvents, but soluble in DMF, DMSO and acetonitrile. The analytical data suggested 1:1 metal : ligand stoichiometry for the complexes. The molar conductance values of the complexes suggested 1:3 electrolytic nature for trivalent metal ion & 1: 2 for divalent metal ion complexes.
Magnetic Moment & Electronic Spectra
The magnetic moment of Fe(III) complex is 5.97 B.M. corresponding to five unpaired electrons and a high spin state of Fe(III) ion. Three bands have been observed in electronic spectrum at 11235, 21740 and 27780 cm-1 corresponding to 6A1g g 4T1g. 6A1g g 4T2g and 6A1g g 4Eg transitions respectively suggesting an octahedral geometry15. The Ti(III) complex shows magnetic moment of 1.69 B.M. for one unpaired electron. The higher value may be due to orbital contribution. A single broad band has been observed at 19230 cm-1 for Ti(III) complex derived from the transition. 2T2g g 2Eg for an octahedral symmetry16. This band is unsymmetrical in shape and is indeed made up of two closely shaped bands. The second band appears as a hump and may be due to the presence of Jahn-Teller distortion in the complex. The UO2(VI) complex as expected for f0configuration is diamagnetic in nature. Magnetic moment for Mn(III) complex is 4.90 mB revealing the high spin nature of the complex corresponding to four unpaired electrons. Electronic spectrum shows a strong band at 20000 cm-1 which can be assigned to ligand to metal charge transfer and a shoulder at 18000cm-1 may be assigned to the 5Eg g 5T2g transition17-18 characteristic of octahedral geometry.
The Co(III) complex is diamagnetic (at 270) as expected for a low spin d6 ion. The electronic spectrum of cobalt (III) complex displays bands at 15450, 21095 and 23370 cm-1. These are similar to those reported for other six coordinated cobalt (III) complexes19 and may be assigned to 1A1gg 3T2g, 1A2gg1T2g and 1A1g g 1T2g transitions respectively.
The electronic spectrum of V(III) complex was recorded in pyridine solution which showed bands at 16000 cm-1 with a shoulder at 21000 cm-1. The low energy band has been assigned to 3T1g g 3T2g whereas the high-energy band to 3T1g g 3T1g (P) transitions respectively, characteristic of octahedral geometry, which is further confirmed by the meff value of 2.88 B.M. for V (III) complex.20
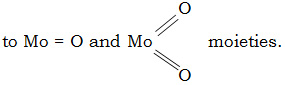
The electronic spectrum of oxomolybdenum (V) complex suggested that the complex may be considered as octahedral with a strong tetragonal distortion resulting from Mo=O bond. The electronic spectrum exhibited three distinct absorption bands in the ligand field region. The low intensity band at 13000 cm-1 in the long wavelength region is possibly due to first crystal field transition 2B2g2E (dxy g dyz, dxz), the second transition at 19000 cm-1 is assignable to 2B2 g B2 (dxy g dx2-y2), the third peak was observed at 30000 assignable to 2B2 g 2A1 (dxy g dz2)21. The magnetic moment of MoO(V) complex is 1.84 B.M.
The electronic spectrum of MoO2 (VI) complex has a single band due to charge transfer transition.
The meff for Ru(III) complex was found to be 1.88 B.M. as expected for an octahedral complex with t2g5 configuration. Its electronic spectrum in chloroform displayed bands at 17860, 21500, 27780 and 33900 cm-1 which may be assigned to 2T2g g 4T1g, 2T2g g 4T2g, 2T2g g 2A2g, 2T1g and charge transfer respectively. These are similar to those reported for octahedral Ru(III) complexes22.
The study of magnetic properties has suggested diamagnetic nature of the Ru(II) complex. The electronic spectrum of the complex in CH2Cl2 shows a band at 22000 cm-1. This band has been assigned to charge transfer transition arising from excitation of an electron from the metal t2g level to unfilled molecular orbitals derived from p* level of the ligand in accordance with the assignment made for similar octahedral Ru(II) complexes.23
Infra Red Spectra
The three N-H stretching frequencies in their spectrum of ligand at 3460, 3360 and 3275 cm-1 were shifted to low frequencies side by 25-50 cm-1. Further amide II absorption [n(C-H) + d(N-H)] around 1500 cm-1 in the ligand spectrum is shifted by 55cm-1. These shifts are of considerable magnitude and confirm that this ligand coordinates through amine and amide nitrogen atom. 24,25,26,27
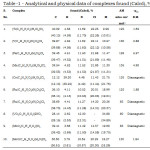 |
Table -1 – Analytical and physical data of complexes found (Calcd), % |
The amide carbonyl absorption n(C=O) remain unaffected in the complex and indicates non coordination of carbonyl oxygen.
These coordination sites are further supported by the appearance of a new band in the far IR region of the complex at 460 cm-1 due to nM-N stretching vibration28.
The analytical data and other evidences support the formation of only 1 : 1 complexes and thus the ligand is quadridentate coordinating through two amide nitrogen atoms and two amine nitrogen atoms.
The coordinated nature of water molecules is suggested by ir and supported by TGA. The complexes showed a non-ligand band in the region of 3480-3510 cm-1 assignable to nOH vibrations, suggesting coordinated nature of water molecules. This is further supported by rocking and wagging modes appearing in the regions of 840-855 cm-1 and 735-750 cm-1 respectively. The thermograms showed inflection point corresponding to the loss of water molecules in the temperature range of 168-2050C.
In UO2 complex bands at 910-930 cm-1 are assigned to n3 vibrations indicating linear character of UO2 group29.
A very strong band found at 940 cm-1 in the spectra of Mo(V) complex corresponds to nMo=O. Also strong bands exhibited by the dioxomolybdenum (VI) complexes at 930 and 900 cm-1 are attributed to nsym O = Mo=O and nasym O = Mo =O respectively of Cis – MoO2 configuration due to the maximum utilization of the available dp arbitals for bonding with the OXO group30.
Conclusion
On the basis of studies performed octahedral geometry has been proposed for all the synthesised complexes, with possible distortion in the case of oxo and dioxo molybdenum complexes due
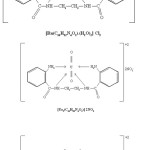 |
Scheme 3 |
References
- Samy CR & Radhey S, Indian J. Chem., 35A, 1 (1996).
- Shen X, Yang QLC and Xie, Synth React Inorg Met Org. Chem, 26, 1135 (1996).
- Kaper ES, Coord Chem Rev. 153, 199 (1996).
- Dietzsch W. Kirmse R & Hoyer E. Coord Chem, Rev. 117, 99 (1999).
- Holm RH, Evenett (Jr) GW & Chakravorty A, Prog. Inorg Chem. 7, 83 (1966).
- Yamada S, Coord Chem. Rev. 1, 415 (1996).
- Curtis NF, Coord. Chem, Rev. 3, 3 (1983).
- S. A. Sadeek, J. Argent Chem. Soc., 93, 165, 2005.T.Benabdallah, A.H. Al-Tair and H. Reffas, S. Afr. J. Chem. 57, 33, 2004. D. Kumar, A. Syamal and A.K. Singh, Indian J. Chem. Sect., A., 42, 280, 2003.
- H.J. Garg and C. Prakash, J. Pharm. Sci., 60, 323, 1971, J. Org. Chem., 35, 1056, 1970.
- C.S. Salazar and M.I. Toral. J. Chil. Chem. Soc. 49, 169, 2004. M.S. Masoud, G.B. Mohamed Y.H. Adbulrazek, A.E. Ali and F.N. Khairy. J. Koream Chem. Soc. 46, 99, 2002.
- F.G. Montelongo, V.G. Diaz and C.R.T. Gonzalez Microchem. J. 27, 194, 1982, F. Kai, Y. Sakanashi, S. Satoh and S. Uchikawa, Anal, Lett., 16(A13), 1013, 1983.
- M.I. Toral, P. Richter, N. Lara, M.T. Escudero and C. Soto Anal, Lett., 33, 93, 2000.
- V. G. Diaz C.R.T. Gonzalez and F.G. MOntelongo, Anales de Quimica, 79, 83, 1983.
- J.J. Arias, F. Jimenez, A.I. Jimenez and F.G. Montelongo, Anales. De Quimica, 79, 248, 1983.
- N.K. Singh and Kushwaha, Indian Journal of Chemistry, Vol.43A, pp.1454-1458, 2004.
- Islam M.S. And Alam M.A., J. Indian Chem. Soc., 76, 2 (1999).
- Kolawola G.A., Synth React to Inorg Met Org Chem 23, 907, 1993.
- Patel I.A., Thaker B.T. & Thaker P.B., Indian J. Chem., 37A, 429, (1998).
- Lever ABP., Inorganic Electronic Spectroscopy (Elsevier Amsterdam) pp. 275-77 (1984).
- Rahul Kumar Rastogi, Poonam Garg and Shamim Ahmad, Asian Journal of Chemistry, Vol. 21, No. 8, 6144-6148 (2009).
- Mohammad Azim and Shamim Ahmad, Oriental Journal of Chemistry, Vol. 27, No. (2), Pg. 673-677 (2011).
- T. Daniel Thangadurai & K. Natarajan, Indian Journal of Chemistry, Vol. 41A, pp. 741-745 (2002).
- P. Viswanathamurthia, R. Karvembub, V. Thahraneeswaranb and K. Natarajan*, J. Chem. Sci. Vol.117, No. 3, pp-235-238 (2005).
- Swamy SJ & Bhaskar K. Indian J. Chem., 38-A, 961(1999).
- Clark RH & Wagner EC, J. Org. Chemistry, 9, 55 (1944).
- Swamy SJ & Kumar BK, Indian J. Chem., 35A, 489 (1996).
- Swamy SJ Kumar BK & Dharmapuri Y, Indian J. Chem., 34A, 811 (1995).
- S.J. Swamy, A Dharma Reddy & K. Bhaskar, Indian Journal of Chemistry, Vol. 40A, pp.1166-1171 (2001).
- Sobhanadevi G & Indrasenan P. Inorg. Chin Acta. 133, 157 (1987).
- Prabhakaran CP & Nair BG, Trans. Met. Chem., 8, 368 (1983).

This work is licensed under a Creative Commons Attribution 4.0 International License.









