Synthesis and Characterization of Hydrotalcite and Hydrotalcite Compounds and their Application as a Base Catalyst for Aldol Condensation Reaction
Baber Masood Bhat
Department of Education, Govt Degree College, Boys Kupwara Kashmir, India
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Previous Studies on MgO doped by alkali cations have shown that solid catalyst are efficient for the aldolization, because of having preferably a high concentration of surface basic hydroxyl groups. Indeed the Mg-Al hydrotalcites actuvated by calcinations at temperatures not exceeding 1073K are mixed oxides of Mg(Al)O type. They show the structure of MgO with smaller crystallographic parameters, then in periclase (MgO). The decrease of the size of the unit cell with increasing Al content can be accounted for by the smaller ionic size of Al3+(0.050 nm), then Mg2+(0.060 nm). These mixed oxides contain both basic sites identified to O2− and OH- in amounts depending upon the calcinations temperature, i.e., is of the dehydroxylation degree of the hydrotalcite and Lewis acid sites provided by Al3+ and Mg2. The existence of acid-base pairs justifies the catalytic properties of hydrotalcites for the Aldolisation. The synthesized Hydrotalcites were then characterized using XRD and TGA/DTA techniques, which confirmed that the synthesized materials are hydrotalcite like compounds. There after the synthesized Hydrotalcites were successfully applied for the typical Aldol Condensation reaction of propionaldehyde and formaldehyde to get desired product methacrolein. We tested catalytic activity of synthesized hyrotalcites and from the results it was clear that, the Mg - Al hydrotalcite catalyzed Aldol reaction propionaldehyde coversion of 57.78% and with selectivity 51.23%to methacrolein. Ni- Al hydrotalcite showed a propionaldehyde conversion of 53.46%with 35.46% selectivity. Co – Al XRD pattern of the formed material confirms that the material is not hydrotalcite like. This material also did not show any catalytic activity for the above said Aldol condensation reaction.
KEYWORDS:Aldolization; Hydrotalcites; Propionaldehyde conversion
Download this article as:| Copy the following to cite this article: Bhat B. M. Synthesis and Characterization of Hydrotalcite and Hydrotalcite Compounds and their Application as a Base Catalyst for Aldol Condensation Reaction. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Bhat B. M. Synthesis and Characterization of Hydrotalcite and Hydrotalcite Compounds and their Application as a Base Catalyst for Aldol Condensation Reaction. Available from: http://www.orientjchem.org/?p=22733 |
Introduction
Hydrotalcite like compounds (HTLC) at present, form a large class of inorganic materials extensively studied as catalysts, anionic exchangers, sorbents and additives. Hydrotalcite mainly comprises of hydroxyl carbonates of magnesium and aluminum and occurs in the form of foliated and contorted plates and/or fibrous masses. The structure of HTLC originates from the packing of layers built up in a manner similar to that found in Brucite, the naturally occurring Mg(OH)2. In brucite the Mg atoms are octahedrally coordinated by six oxygen atoms belonging to six – OH groups, each –OH group is in turn shared by three octahedral cations and points to the interlayer space Cavani and Vaccar 1991). When some of the Mg(II) cations are isomorphously replaced by Al(III) cations, carbonate anions are inserted in between the metal hydroxide sheets so as to maintain the electro neutrality. As a result hydotalcite with ideal formula, Mg6Al2(OH)16CO3.4H2O is obtained. Even though hydrotalcite and hydrotalcite like minerals are found in nature, it is relatively simpler and cheaper to prepare these compounds on a laboratory scale (1,2).
The general formula of synthetic HTLC (Hydrotalcite like compounds) is written as [M(II)1-x M(III)x (OH)2]x+ [An-x/n]x-m(S) where M(II) may be Mg, Cr, Fe, V, Co etc, An- is the charged compensating anions (CO32-, SO42-, Cl–, NO3– Organic anions , etc), m the number of moles of co-intercalated solvent (S), generally water per formula weight of compound. The M(III) metal mainly Al, Cr, Mn, etc. the number of moles x of M (III) cation per formula weight of compound, generally ranges between 0.2 & 0.3 and its value determines the charge density of the layer. The objective of the present work is to synthesize hydrotalcite and hydrotalcite like compounds, their application as a base catalyst for Aldol condensation reaction.
Materials and Methods
Synthesis of Hydrotalcites :-
Synthesis of Hydrotalcite was done by using Co- precipitation method from homogeneous solution.
Synthesis of Mg – Al Hydrotalcite:
Charge for Mg – Al hydrtalcite:
Solution A:
Mg (NO3)2 – 15.0 g (0.0596 mole)
Al (NO3)3 – 8 .77 g (0.0234 mole)
Solution B:
NaOH – 7.56 g (0.189 mole)
Na2CO3 – 5 .84 g (0.055 mole)
Procedure for synthesis of Hydrotalcite
Solution A was prepared by taking 15 g (0.0596 mol) of Mg (NO3)2 and 8.77 g(0.0234 mol) of Al(NO3)3 dissolved in 60 ml distilled water. Solution B was prepared in a 3-neck round-bottomed flask by taking 7.56 g (0.189 mol.) of NaOH and 5.84 g (0.055 mol.) of any Na2CO3 dissolved in 250 ml distilled water. The solution A was then added slowly to the solution B under constant vigorous stirring (using over head stirring shown in figure 4). The addition was done over a period of 3 hours. The contents were then heated to 338K (650 C) and maintained at this temperature for 6 hours (3).
The precipitate formed was filtered and washed with hot distilled water until the pH of the filtrate was 7. The precipitate was then dried in the oven at 353K (800C) for 15 hours.
 |
Figure 1: The setup for the Hydrotalcite preparation |
Activation of the Hydrotalcites
Hydrotalcite was activated by carrying out the calcinations at 723K under a flow of air. The temperature was raised at the rate of 10K per minute to reach 723K and maintained for 8 hours. This solid was then cooled in dry nitrogen and rehydrated at room temperature under a flow of nitrogen gas saturated with water vapour. The flow of wet nitrogen was maintained at 6L per hours.
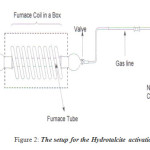 |
Figure 2: The setup for the Hydrotalcite activation. Click here to View figure |
Working on the similar lines Ni-Al and Co-Al hydrotalcites were also prepared.
Charge of Ni-Al Hydrotalcite:
Solution A:
Ni (NO3)2 -17.332 g (0.0596mole)
Al (NO3)3 – 8.77 g (0.0234 mole)
Solution B:
NaOH – 7.56 g (0.189 mole)
Na2CO3 – 5.84 g (0.055 mole)
Charge of Co – Al Hydrotalcite:
Solution A:
Co (NO3)2 – 17.025 g (0.0596 mole)
Al (NO3)3 – 8. 77 g (0.0234 mole)
Solution B:
NaOH – 7.56 g (0.189 mole)
Na2CO3 – 5. 84 g (0.055 mole)
Condensation Reactions
The condensation reactions of aldehydes, as well as of ketones are widely used in organic synthesis, mainly because they lead to C – C bond formation.
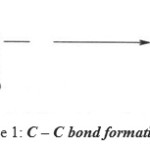 |
Scheme 1: C–C bond formation reaction. |
These reactions are typically catalyzed by bases, but can also proceed over acid catalyst. Of course, the aldehydes (ketones) can be the same or different
In the case of aldol condensation of aldehydes, when the aldehydes are different, two path ways may be involved namely the self and cross condensation reactions, as in the case of condensation of propionaldehyde with formaldehyde.
Self condensation reaction:
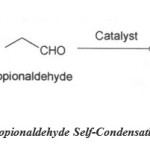 |
Scheme 2: Propionaldehyde Self-Condensation Reaction. |
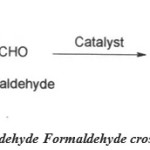
Cross condensation reaction
 |
Scheme 3: Propionaldehyde Formaldehyde Cross Condensation reaction. Click here to View figure |
We can note that besides the Aldol condensation products, depending on the nature of active sites, the aldehydes can be involved into Cannizzaro and other reactions, which enlarge the range of the first generation of products. In the second stage various condensation, polymerization and other reactions can take place.
An aqueous solution of formaldehyde was used as one phase and propionaldehyde in toluene, as an organic phase. Because of physical property of hydrotalcite it will remain in aqueous phase and rarely get expose to organic phase thus minimizing self – Aldol condensation of propionaldehyde as the propionaldehyde is sparingly soluble in water, the chances of self – Aldol condensation will be minimized since formaldehyde is in bulk quantity cross condensation will be favoured. Thus, this two – phase system will selectively catalyze cross aldol condensation to yield methacrolein.
Methacrolein is an important large volume intermediate, used in the synthesis of methyl Methacrylate, (4) which is an important monomer for specialty polymers (5).
Application of Synthesized Hydrotalcites
Reaction Scheme
 |
Scheme 4: Propionaldehyde Formaldehyde cross Condensation Reaction. |
Reaction Procedure
3 ml Propionaldehyde is added to a 50 ml round bottomed flask containing 8 ml toluene. This mixture is stirred and 0.2 ml reaction mixture is removed from it as an initial sample. In this mixture then 5 ml formaldehyde and 0.200 g Hydrotalcite are added. The round-bottomed flask is then connected to a reflux condenser and then whole assembly is flushed with N2. The reaction mixture is then immersed in an oil bath maintained at 600 C by a heating coil connected to a temperature controller. The reaction is carried out for 6 hours. The round-bottomed flask is then removed from the oil bath and cooled to a 150 C by keeping it in an ice bath. The reaction mixture is then discharged, and the toluene layer is separated from formaldehyde layer. Both the layers are then analyzed separately on Gas chromatography.
Results and Disscussion
Characterization of Hydrotalcites and hydrotalcite like compounds:
All hydrotalcites were characterized by using XRD and TGA techniques.
Mg – Al Hydrotalcite:-
 |
Figure 3: Powder XRD Pattern Mg–Al hydrotalcite. Click here to View figure |
 
Powder X – ray diffraction patterns were recorded for Mg – Al hydrotalcite on RIGAKU MINI FLEX instrument from (2θ = 50 to 800). The diffraction patterns obtained were exactly similar to that of typical Mg – Al hydrotalcite diffraction patterns.
 |
Figure 4: TG/DT analysis of Mg – Al Hydrotalcite Click here to View figure |
Weight of sample = 4. 626 Mg
Temperature range = 308K to 758K
Heating rate = 100C/min
Atmosphere = static air
Reference = α – Alumina
Table 1: Thermal analysis results.
| Sample | First Weight loss (%) | Temperature (K) | Second weight Loss (%) | Temperature (K) |
| Mg–Al | 11.98 | 488.9 | 36.74 | 713.0 |
TG analysis results for Mg – Al (2.5 ratios) Hydrotalcite
The TGA data reported in the table above showed two stages of weight loss, which is a characteristic property of hydrotalcite like compounds. The first weight loss is observed between 355K to 490K, due to the loss of absorbed water molecules, which is followed by loss of interlayer water molecules. The second weight loss being between 500K to 713K, is due to dehydroxylation of the cation layers and the removal of carbonate via the formation of carbon dioxide from the brucite sheet.
DT analysis results
The first weight loss is observed between 353K to 490K. There is a broad endothermic peak related to the dehydration of the sample. The second endothermic peak corresponds to the weight loss due to de hydroxylation and de carbonation reactions. This is matching with the reported values in the literature.
Ni – Al Hydrotalcite
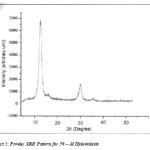 |
Figure 5: Powder XRD Pattern for Ni – Al Hydrotalcite. Click here to View figure |
Powder X – Ray diffraction patterns were recorded for Ni – Al hydrotalcite on RIGAKU MINI FLEX instrument from (2θ = 50 to 800). The diffraction patterns obtained were exactly similar to that of typical Ni – Al hydrotalcite diffraction patterns.
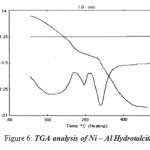 |
Figure 6: TGA analysis of Ni – Al Hydrotalcite Click here to View figure |
Weight of sample = 3.433 mg
Temperature range = 308K to 758K
Heating Rate = 100C/m
Atmosphere = Static air
Reference = α – Alumina
Table 2 : Thermal analysis results
|
Sample |
First Weight Loss (%) | Temperature (K) | Second Weight Loss (%) | Temperature (K) |
| Ni – Al | 12.97 | 487 | 36.20 |
715 |
TG Analysis results for Ni – Al (2.5 ratios) Hydrotalcite
The TGA data reported in the table above showed two stages of weight loss, which is a characteristic property of hydrotalcite like compounds. The first weight loss observed between 359K to 487K, is due to the loss of absorbed water molecules, which is followed by loss of interlayer water molecules. The second weight loss observed between 490K to 715K is due to the dehydroxylation of the cation layers and the removal of carbonate via the formation of carbon dioxide from the brucite sheet (6).
DT analysis results
The first weight loss is observed between 359K to 487K there is broad endothermic peak related to the dehydration of the sample, where as the second endothermic peak corresponds to the weight loss due to dehydoxylation and removal of carbon dioxide from the carbonate anion present in the brucite layer, which is matching with the reported values.
5.1.3 Co – Al Hydrotalcite
Powder X – Ray diffraction Pattern was recorded for Co – Al hydrotalcite on RIGAKU MINI FLEX instrument from (2θ = 50 to 800). The diffraction Patterns obtained were not matching with the reported pattern in the literature. This indicates that the hydrotalcite structure has collapsed during calcinations.
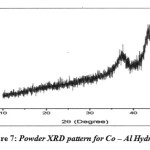 |
Figure 7: Powder XRD pattern for Co – Al Hydrotalcite Click here to View figure |
Gas Chromatograph (GC) is a modern technique for the analysis of vapourizable samples. In GC the sample is vapourized and injected on to the head of a chromatographic column. Elution is brought about by the flow of inert gases mobile phase. In contrast to most other types of chromatography, the mobile phase does not interact with molecules of the analyte; its only function is to transport the analyte through the column(7). 
Analysis:
The Details of the analytical method are
Column – 5% Phenyl methyl Siloxane
Carrier Gas – Helium
Detector – FID
Fuel – H2
Oxidant – Air
Oven Programming
400C for 4 minutes
250C/min ramp – 1500 C for 10 mins
250C/min ramp – 2900C for 10 mins
Column Programming
10 Psi for 4 min.
10 Psi/min ramp – 25 Psi till complete analysis.
Using above said analytical method the reaction mixtures were analyzed on gas chromatograph (GC).
The Typical chromatogram of the final reaction is given below:
| Hydrotalcite | % Conversion of Propionaldehyde | Selectivity to Methacrolein | Selectivity to 3 – Hydroxy – 2 – Methylpropanal |
| Mg – Al (A) | 57.78 | 51.23 | 27.80 |
| Ni – Al (B) | 53.46 | 45.46 | 25.73 |
| Co – Al (C) | 2.31 | 0.0 | 0.0 |
From the results it is clear that the Mg-Al hydrotalcite catalyzed the Aldol reaction with propionaldhyde conversion of 57.78% and with the selectivity 51.23% to methacrolein. Ni-Al showed a propanaldhyde conversion of 53.46% with 35.46% selectivity.
References
- Kukkadapu, Ravi K.; Witkowski, Marc S.; and Amonette, James E., “Synthesis of a Low-Carbonate High-Charge Hydrotalcite-like Compound at Ambient Pressure and Atmosphere” (1997). US Department of Energy Publications. Paper 145. http://digitalcommons.unl.edu/usdoepub/145.
- Othman, M. R., Helwani, Z., Martunus and Fernando, W. J. N. (2009), Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: a review. Appl. Organometal. Chem., 23: 335–346. doi: 10.1002/aoc.1517.
- Roelofs, J.C.A.A A.J.Van Dillen, K.P. De Jong; (2000) Base-catalyzed condensation of citral and acetone at low temperature using modified hydrotalcite catalysts Catalysis Today, 60 297 – 303.
- Cavani F., F. Trifirb, Vaccar A. (1991). Hydrotalcite-type anlonlc clays: Preparation, properties and applications. coldysis T&y, I I ( I99 I ) 173-301.
- Cocheci, L. P. Barvinschi R. Pode, E. Popovici and E.M. Seftel ( 2010)Structural Characterization of Some Mg/Zn-Al Type Hydrotalcites Prepared for Chromate Sorption from Wastewater. Chem. Bull. “POLITEHNICA” Univ. Volume 55(69), 40-45.
- Maria A. Aramendia, Victoriano Borau, Cesar. Jimenez, Josem.Marinas, Jose R. Ruiz and Francisco J. Urbano; Catalytic transfer hydrogenation of citral on calcined layered double hydroxides Applied Catalysis, Issue 1 (2001) 95 – 101.
- Choudhary Vasant R, Rani Jha And Pankaj A Choudhari Highly active and reusable catalyst from Fe-Mg-hydrotalcite anionic clay for Friedel–Crafts type benzylation reactions. J. Chem. Sci., Vol. 117, No. 6, November 2005, pp. 635–639.

This work is licensed under a Creative Commons Attribution 4.0 International License.









