Studies on the Solvent Extraction of Iron (III) with Tri-iso-octylamine from Aqueous Mineral Acid Solutions
A.V.L.N.S.H. Hariharan1, Ch. Sudhakar1 and B. Venkateswara Rao2
1Department of Chemistry, GIT, GITAM University. Visakhapatnam - 530 045, India.
2Department of Engineering Chemistry, Andhra University. Visakhapatnam - 530 003, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Liquid-Liquid extraction of iron (III) was carried out with 5.0×10-2 M of Tri iso-octylamine in benzene from hydrochloric, sulphuric, nitric and perchloric acid. After extraction from the organic phase, iron was stripped with 1M H2SO4 and was determined spectrophotometrically at 480nm as thiocyanate complex. The extraction was quantitative only in the presence of hydrochloric acid solution and are partial form sulphuric, nitric and perchloric acid solutions. Based on the results obtained in this study, estimation of iron in food samples and alloys has been attempted.
KEYWORDS:Liquid; Liquid Extraction; Iron (III); Tri iso-octylamine (TIOA); Mineral acid; Food samples; Iron alloys
Download this article as:| Copy the following to cite this article: Hariharan A. V. L. N. S. H, Sudhakar Ch, Rao B. V. Studies on the Solvent Extraction of Iron (III) with Tri-iso-octylamine from Aqueous Mineral Acid Solutions. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Hariharan A. V. L. N. S. H, Sudhakar Ch, Rao B. V. Studies on the Solvent Extraction of Iron (III) with Tri-iso-octylamine from Aqueous Mineral Acid Solutions. Available from: http://www.orientjchem.org/?p=22748 |
Introduction
Iron is one of the most essential elements in the human body. People with insufficient iron will develop some illnesses such as iron deficiency anemia (IDA), stomatitis, chronic gastritis, etc.. Iron deficiency anemia is one of the world’s most common nutritional deficiency diseases. Evidence has been presented that at low levels iron is an essential element in the diet, whereas at higher concentrations it is toxic 1. Because of the different biological roles of iron in humans, animals, plants, and oceans, the need for analysis of iron in environmental and biomedical materials have been increased. The extraction of iron in its trivalent form from aqueous hydrochloric2-6 and sulphuric acid7,8 solutions by various amines has been studied. Earlier, we have reported extraction of iron(III) with TOA in chloroform from hydrochloric, sulphuric and nitric acid solutions9.
However, there were no studies reported in literature on the extraction of iron (III) with Tri iso-octylamine (TIOA) or their suitable derivatives . Therefore this paper presents an account on systematic investigations of extraction of Iron (III) with Tri iso-octylamine (TIOA) in the presence of mineral acids. The applicability of the method was extended for separation of Iron (III) in food samples and iron alloys.
Experimental
Apparatus and Reagents
An ELICO SL 191 UV-Visible Double beam Spectrophotometer with matched 10 mm corex glass cuvetts were used to determine iron content.
A stock solution of Iron (III) was prepared by dissolving 482.25 g/mol of Ammonium iron(III) sulfate (E.Merck) in 500 ml of double distilled water. The solution was standardized volumetrically with potassium dichromate using diphenyl amine as the indicator. A diluted solution of iron (III) of appropriate concentration was prepared from the stock solution. A solution of 5.0X10-2 M TIOA in benzene is used for throughout the extraction.
General Procedure for Iron (iii) Extraction
An aliquot (10ml) of a solution containing iron (III) was taken and was added with appropriate concentration of mineral acid. The resulting solution was transferred to a separating funnel and 10 ml of 5X 10-2 M of TIOA was added to it. The solution was vigorously shaken for five minutes. The two phases were allowed to settle and separate. Iron (III) from the organic phase was stripped with 10 ml of 1M H2SO4 and was determined spectrophotometrically10 at 480 nm as its colored complex with thiocyanate. The concentration of Iron (III) was computed from the calibration curve.
Results And Discussion
Extraction as a function of Acidity
Iron (III) was extracted from different mineral acids (HCl, H2SO4, HNO3 and HClO4) with 5.0X10-2 M TIOA in benzene and the results are presented in Table-1. The variation of distribution ratio as a function of aqueous phase concentration of the acid (HCl H2SO4, and HClO4) solutions increased sharply with increasing concentration of the acid. From hydrochloric acid solutions, the distribution ratio (Kd) increased with increasing the concentration of the acid up to 8.0 M (Maximum extraction of 98.21%) and remained constant up to 10.0 M acidity. The extractions are nearly quantitative (Table 1). On the other hand the extraction of iron (III) from sulphuric, nitric and perchloric acid solutions by TIOA in benzene as a function of acidity, the distribution ratio (Kd) gradually increased with increasing the concentration of the acid up to 10.0 M(maximum at 80.32% ), 12.0 M (max. 79.12% ) and 12.0 M (max. 68.16% ) respectively.
Composition of the Extracted species
The extraction isotherm method 11 and distribution ratio method 12 were employed to determine the composition of the extracted species. In the extraction isotherm method the limiting ratio of the metal to TIOA was found unity with all the acid systems. Representative data from hydrochloric acid solutions has been provided in Table-1.
Table 1: Extraction as a function of varying Acidity
[Fe(III) ]= 1.0 x 10-3 M
[TIOA] = 5.0 x 10-2 M
|
HCl |
H2SO4 |
HNO3 |
HClO4 |
|
|
Molarity(M) |
%Extraction |
%Extraction |
%Extraction |
%Extraction |
|
1.0 |
55.99 |
37.39 |
30.05 |
25.65 |
|
2.0 |
82.82 |
50.98 |
39.95 |
29.95 |
|
3.0 |
91.77 |
58.50 |
44.45 |
37.39 |
|
4.0 |
94.71 |
63.51 |
51.50 |
44.54 |
|
5.0 |
95.71 |
65.44 |
57.51 |
53.46 |
|
6.0 |
96.42 |
68.16 |
60.44 |
58.50 |
|
7.0 |
97.42 |
72.24 |
63.16 |
61.00 |
|
8.0 |
98.21 |
76.02 |
68.24 |
64.05 |
|
9.0 |
98.21 |
79.12 |
72.42 |
65.44 |
|
10.0 |
—— |
80.32 |
76.64 |
66.15 |
|
11.0 |
—— |
80.32 |
76.95 |
67.16 |
|
12.0 |
—— |
—— |
79.12 |
68.16 |
Effect of Reagent Concentration
With all other factors being kept constant, iron (III) was extracted with 10 ml of TIOA with concentration, varying from 1.0 X 10-2 M to 4.5 X 10-2 M. The log-log plots of Kd Vs TIOA from various acid solutions gave straight lines of with unit slope expect sulphuric acid media (solvation number is two). Fig-1 corresponds to plot from hydrochloric acid media.
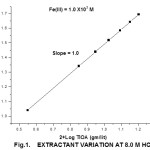 |
Figure 1: Extractant Variation at8.0 HCi Click here to View figure |
Effect of Diluents on Extraction
Several solvents with varying dielectric constants were tested as the diluents (Table -2). Quantitative extractions was achieved with benzene as diluent. More than 80% efficiency was obtained with hexane, carbon tetrachloride, xylene, toluene, cyclo hexane and benzene. Nitrobenzene and n-heptane were found to be poor in extraction. Hence benzene was preferred as diluent throughout the study.
Table 2: Effect of various diluents on extraction( HCl medium)
[Fe(III)] = 1.0 x 10-3 M [TIOA] = 5.0 x 10-2 M
|
Diluent |
Dielectric constant |
% extraction |
| Benzene |
4.81 |
98.21 |
| CHCl3 |
2.28 |
92.24 |
| CCl4 |
2.23 |
90.86 |
| Cyclo hexane |
2.00 |
81.33 |
| n-Hexane |
1.89 |
83.57 |
| n-heptane |
1.92 |
74.72 |
| Nitrobenzene |
34.82 |
69.25 |
| Toulene |
2.43 |
84.39 |
| Xylene |
2.56 |
87.53 |
Effect of various stripping agents
After extraction, iron(III) was stripped with 20ml reagents of various concentrations (0.1 – 1 .0 M) of HCl, HNO3, ACOH, NaOH and H2SO4 solutions.. It was observed that 1.0 M H2SO4 alone is a good stripping agent. However in no case the acid strips out all the iron (III) in a single extraction. 99.8% iron (III) could be recovered from organic phase by making contact three times with equal volumes of 1.0 M H2SO4.
Absorption spectra
The individual Iron (III) extracted species with TIOA was studied in U.V. region13,14. The absorption spectra from sulphuric acid media exhibits absorption band at 295 and 355 nm . These two are the absorption characteristics of Fe(OH) and hydroxyl – group bridging species Fe(OH)2 Fe respectively, and the appearance of new peak at 305 nm corresponds to the complexes FeSO4+ and Fe(SO4)-2.
The observed iron: TIOA molar ratio of two from sulphuric acid media and unity from other acid solutions (by distribution ratio method) could be explained as arising from the extraction of iron (III) by the following ion-exchange mechanism.
From hydrochloric( Smulek15) nitric and perchloric acid solutions:
TIOAHCl + Fe3+ + 4Cl– [TIOAH+ FeCl4–] org + Cl–
TIOAHNO3 + Fe3+ + 4NO3– [TIOAH+ Fe (NO3)4-] org + NO3–
TIOAHClO4 + Fe3+ + 4ClO4– [TIOAH+ Fe (ClO4) 4-] org + ClO4–
From sulphuric acid solutions:
2(TIOA)2SO4 + 2Fe3+ + 2SO42- + 2H2O(aq) [(TIOAH)2SO4.FeOH(SO4)2 ]2 org + 2H+
On the basis of the proposed mechanism for the extraction of iron (III), the dependence of the distribution ratio on the nature of the mineral acid was well under stood.
Analysis of iron in various samples
The validity of the method of extraction for recovery of iron has been tested by analyzing food samples and iron alloys. The samples were weighed accurately (0.5 – 1.0 gm) and finely powdered in a mortar. A known weight of the powdered sample was dissolved in an aliquot of aquaregia. The solution was evaporated and extracted with dilute hydrochloric acid solution. The mixture was shaken well for about 15 min. Then the mixture was diluted by 0.01 M HCl solution to the mark and then filtered by Whatmann filter paper No. 40. The first portion of filtrate was discarded. The clear solution obtained was made up to 100 ml and used as stock solution. 10 ml of this iron solution was shaken for five minutes with an equal volume of 5.0X 10-2 M of TIOA. After separation of two phases, Iron (III) from the organic phase was stripped with 10 ml of 1.0M sulphuric acid and was determined spectrophotometrically as described earlier. Results are presented in Tables -3&4.
Table 3 : Analysis of Iron in Food Samples
| Sample |
% of Fe (III) Present |
% of Fe (III) Found | % Recovery |
| Ragi |
3.00 |
2.84 |
94.66 |
| Green gram |
4.05 |
3.98 |
98.27 |
| Soya beans |
20.0 |
19.6 |
98.0 |
| Dextrin |
100.22 |
99.42 |
99.20 |
Table 4: Determination of Iron in Alloys.
|
Material |
Carbon |
Manganese |
Sulfur |
Phosphorus |
Silicon |
Iron |
Amount of Iron(III) taken (ppm) |
Amount of Iron(III) found (ppm) |
% Recovery |
|
Cast Iron |
3.430 |
0.880 |
0.041 |
—- |
2.120 |
91 – 91.2 |
91.1 |
90.9 |
99.78 |
|
Pig iron |
3.5-4.5 |
0.5-2.5 |
0.018-0.1 |
0.03-0.1 |
0.25-3.5 |
91-94 |
92.0 |
91.6 |
99.56 |
|
Carbon steel |
0.007-1.3 |
0.3-1.0 |
0.02-0.06 |
0.002-0.1 |
0.005-0.5 |
98.1-99.5 |
99.3 |
99.1 |
99.79 |
|
Wrought iron |
0.05-0.25 |
0.01-0.1 |
0.02-0.1 |
0.05-0.2 |
0.02-0.2 |
99-99.8 |
99.5 |
99.1 |
99.59 |
Conclusion
The proposed method is simple, rapid and selective. It does not take more than half an hour to extract and determine iron (III) content. The method has practical utility as iron(III) can be separated from manganese, copper, cobalt, cadmium and zinc (under the experimental conditions) which are generally associated with it in alloys and minerals.
Acknowledgements
Thanks are due to Dr. V. Muralidhara Rao, Retd. Professor, School of Chemistry, AndhraUniversity, Visakhapatnam for his valuable suggestions. Thanks are also due to Principal, GIT and Management of GITAM University for providing necessary facilities
References
- Ghadamali B, Mansour A C and Zeinab B, Eurasian J. Anal. Chem., 4(3), 285-293.(2009) .
- Sahu, K.K and Das, R.P. , Metlrgy. Met. Trans.B, 31(B), 1169.(2000) Lee, MS Lee..K.J Sepn. of Iron by extraction, , Hydrometallurgy, 80, 163.(2005).
- Staszak K, Clerpiszewski R and ProchaskaK, Polish J. Chem. Tech., 1(1), 1-5. (2011).
- Oren, J.J Gough, KM Gesser HD , Canadian J. chem., 57, 2032. (1979).
- Gupta,B. Deep,A. Singh,V. Tandon,SN. , Hydrometallurgy, 70, 121.( 2003).
- Cattrall RW and West BO. , J. Inorg .Nucl .Chem ., 28, 3035. (1966) .
- Alguacil, FJ and Amer,S. , Polyhedron, 6(11), 1755.( 1986).
- Hariharan. A.V.L.N.S.H, Sudhakar. Ch, and Venkateswara Rao B., Asain. J. Res. Chem., 5(2), 245-247.( 2012).
- Vogel, A.I., “A Text book of quantitative Inorganic Analysis” 3rd Edition, 1962, Longman, London.
- Coleman. CF., Brown. KB., Moore.JG., and Allen. K.A., Proc.2nd Intl. Conf., Peaceful uses of Atomic Energy, Geneva, C.10, 510. (1958).
- Hesford.E. and Mckay.H.A.C., Trans Faraday Soc., 54, 573. (1958) .
- Akinobu N, Hitoshi T, Yasunobu O and Sekine T. Anal. Sci., 15, 177. (1999).
- Campederros, M.E., Marchese, J., Journal of Membrane Science, 164, 205.( 2000).
- Smulek W and Siekjerski S, j. Inorg. Nucl. Chem. 24, 1651 (1962).

This work is licensed under a Creative Commons Attribution 4.0 International License.









