Structure and Properties of Aluminum Alloys with Cerium, Praseodymium and Neodymium
Mohammad Razazi Boroujeni1 , Reza Amini Najafabadi2, Bakhtior Eshov Badalovich3 , P. Pulatov3 and Abdulkhair Badalov Badalovich4
1Department of Material Science And Engineering, Isalmic Azad University, Majlesi Branch, Isfahan, Iran. 2Department of Material Science and Engineering, Isalmic Azad University, Najafabad branch, Isfahan, Iran. 3Chemistry Institute, Tajikistan Academy of Sciences, Dushanbe, Tajikistan. 4Department of Chemical Technology and metallurgy, Tajik Technical University named after academician M.S.Osimi, Dushanbe, Tajikistan.
Chemical compositions and microstructure of Al – Ln (Ce, Pr, Nd) alloys and intermetallic compounds have been studied. The laws of change of thermophysical properties and thermodynamic functions of temperature according to composition of the alloys have been achieved. It was revealed the regularity of their changes depending on the composition. Melting temperature of intermetallic compounds of the system has been specified by Semi empirical method.
KEYWORDS:Microstructures; Thermodynamic properties; Alloys
Download this article as:| Copy the following to cite this article: Boroujeni M. R, Najafabadi R. A, Badalovich B. E, Pulatov P, Badalovich A. B. Structure and Properties of Aluminum Alloys with Cerium, Praseodymium and Neodymium. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Boroujeni M. R, Najafabadi R. A, Badalovich B. E, Pulatov P, Badalovich A. B. Structure and Properties of Aluminum Alloys with Cerium, Praseodymium and Neodymium. Available from: http://www.orientjchem.org/?p=11923 |
Introduction
Progress in many areas of science, engineering and technology practically possible in the presence of reliable information used in the study, design, and operation of materials and products. It is important to know the properties of individual structural components of alloys, and the results of their collegiate influences [1].
Heat capacity is one of the most important physical properties of solids, characterize the change of state of matter with temperature. The study of the heat capacity is one of the main research methods of structural and phase transformations in alloys. Other physical characteristics of solids can be defined from the temperature dependence of the specific heat: the temperature and the type of phase transformation temperature Debye energy of vacancy, the coefficient e heat capacity, and other thermodynamic functions. [2-4].
Experimental part
This paper presents the results of studies of the structure, thermal properties and thermodynamic functions of aluminum alloys containing 0.05, 0.1, 0.5, wt. % Cerium, praseodymium and neodymium. Thermophysical properties were in the temperature of ranges about 293-873 ◦C. Aluminum grade A99, Ce – Ce EO TU 48 – 295 -85, Pr – Pr M -1 -4-215 TU 48 – 72, Nd – H M -2 TU48 – 40 -205 – 72 were used to study the research.
The sample consists of a cylinder shape with 30 mm height and 16 mm diameter with drilled canal at one end, into the thermocouple. Thermocouples summed to gauge Digital Multimeter UT 71B, which produces direct fixation test results on a computer in a table. Temperature accuracy was 0.1 °C. The processing of the results of measurements were performed using the MS Excel. Dependence of the cooling temperature (T) of the sample and then ( ): T=f( ) were constructed using Sigma Plot. Regression coefficient was not lower than 0.998.
Results And discursion
Chemical composition of the alloys is determined by scanning electron microscope SEM series AIS 2100 (Korea). As an example, Figure 1 shows the intensity diffraction lines of the components aluminum alloy containing 0.5 wt. % Cerium. Difference between the specified composition and received information is little.
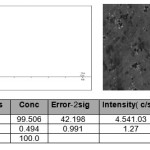 |
figure :1 The intensity of the diffraction lines of the components of aluminum alloy containing 0.5 % Cerium. Click here to View figure |
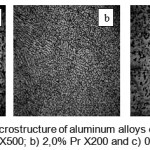 |
figure :2 The microstructure of aluminum alloys containing masses. %:a) 0.5% Ce – X500; b) 2,0% Pr X200 and c) 0.05% Nd X500 Click here to View figure |
Microstructure of Alloys which were taken on a Canon microscope, has certain direction, fine and uniform, indicating that improved mechanical properties. Within the investigated compositions, structure of alloys consists of a solid solution
α – Al + eut. (α – Al + Al 11 Ln 3.) As the concentration of lanthanide share including the said eutectic aluminum solid solution increases (Fig. 2).
igure 3. shows the temperature dependence of the specific heat of aluminum alloys with cerium and neodymium. Increasing temperature, regardless of the composition of the observed increase in the specific heat as aluminum and its alloys. Relative influence of the concentration of lanthanide on the specific heat capacity, observed slight reduction of the latter. This pattern is typical for aluminum alloys with praseodymium.
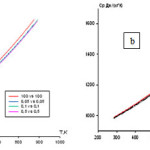 |
figure :3 The temperature dependence of the specific heat of aluminum alloys with cerium (a) and Nd (b).a Click here to View figure |
The temperature dependence of the cooling rate of aluminum alloys with praseodymium have been shown in figure 4. It shows the cooling rate of the alloys containing 0.05 wt.% Praseodymium metal with aluminum. Increasing addition of praseodymium, cooling rate increases.
Tables 1-3 show the values of the temperature dependence of the enthalpy, entropy and Gibbs energy for aluminum alloys with cerium, praseodymium and neodymium, respectively.
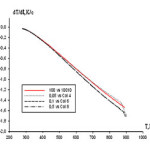 |
figure :4 Temperature dependence of the cooling rate of aluminum alloys and with praseodymium. Click here to View figure |
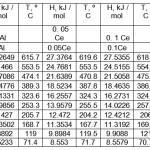 |
Table 1: The temperature dependence of the enthalpy of cerium-aluminum alloys. Click here to View table |
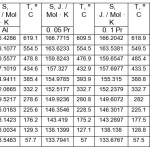 |
Table 1:The temperature dependence of the entropy of aluminum alloys with praseodymium. Click here to View table |
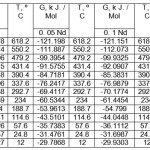 |
Table 1: The temperature dependence of the Gibbs energy of aluminum alloys with neodymium. Click here to View table |
As can be seen on the tables at an elevated temperature is not dependent on the composition of all alloys is observed rise of the thermal properties. Temperature range which studied in the smallest value of the specific heat is observed in aluminum alloys with cerium. Depending on the atomic number of the lanthanides, from cerium, praseodymium and neodymium an increase in the value of the specific heat is observed. According to the Tables, the investigated metals reduce the value of the temperature dependence of the entropy and enthalpy of aluminum.
Conclusions
Studying the microstructure of alloys, it is found that the structure of alloys consists of a solid solution of α – Al + eut. (α – Al + Al 11 Ln 3) and by the method of cooling the temperature dependence specific heat capacity, entropy and enthalpy of aluminum alloys have been determined. It was found that increasing temperature regardless of an increase in the specific heat as aluminum and its alloys. The influence of the concentration of lanthanide on the specific heat is characterized by a slight decrease in the entropy and enthalpy of aluminum alloys.
References
- Zinoviev V. Thermophysical properties of metals at high Temperatures, Moscow, Metallurgy, 1989, 384 p.
- Zelikman A., Meyerson, GN Metallurgy of Rare Metals. – Metallurgy, 1973.-608s.
- Teylor C. Intermetallic compounds of rare-earth metals.-M., New York, 1974, 224
- Spedding D., Dahan A. Rare-earth metals. -M., New York, 1965.-324p.
- Rohlin LL Magnesium alloys containing rare earth metals. M.: Science, 1980., 188p.
- Ugai YA General and inorganic chemistry. -M: High. School., 2004, 527p.
- Kubashevsky O. Olkkok SB Metallurgical thermochimiya.-AM-M.: Metallurgy, 1982., 280p.
- Eight. VA Lebedev, VI Kober, LF Yamschikov Thermochemistry of alloys of rare earth and actinide metals. – Chelyabinsk, Metallurgy, 1989, 366p.
- Mondolfo LF Structure and properties of aluminum alloys. M. Metallurgy, 1979, 639 p.
- Elliot RP, The structures of binary alloys. Moscow: Metallurgy. In 1970. T. 472p.

This work is licensed under a Creative Commons Attribution 4.0 International License.









