Simultaneous Estimation of Cefixime and Moxifloxacin in Bulk and its Pharmaceutical Dosage form by RP-HPLC
G.S. Devika1*, M. Sudhakar1 and J. Venkateshwara Rao2
1Department of Pharmaceutical Chemistry, Malla Reddy College of Pharmacy, Maissamaguda, Dullapally, Secunderabad -14, India. 2Departmentof Pharmaceutical Chemistry, Sultan Ul Uloom College of Pharmacy, Banjara Hills, Secunderabad - 500 034, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
A simple, efficient and reproducible reverse phase high performance liquid chromatographic method was developed and validated for the Simultaneous determination of cefixime (CEF) and moxifloxacin (MOX) in combined dosage form. Chromatographicseparationofthetwo drugswasperformedonaPurospherBDSC18 column(250mm×4.6mmid, 5µm particlesize). The mobile phase comprising of acetonitrile and 0.01M KH2PO4 in a ratio of 40:60 v/v at a flow rate of 1.0ml/min. The detection was made at 276 nm. The retention time of cefixime and moxifloxacin was found to be 3.140±0.007min and 7.007± 0.006min. Calibration curve was linear over the concentration range of 20-120 µg/ml for both cefixime and moxifloxacin .All the analytical validation parameters were determined and found in the limit as per ICH guidelines, which indicate the validity of the method. The developed method is also found to be precise, accurate, specific, robust and rapid for the simultaneous determination of cefixime and moxifloxacin in tablet dosage forms.
KEYWORDS:Cefixime; Moxifloxacin; Method development and validation; RP-HPLC
Download this article as:| Copy the following to cite this article: Devika G. S, Sudhakar M, Rao J. V. Simultaneous Estimation of Cefixime and Moxifloxacin in Bulk and its Pharmaceutical Dosage form by RP-HPLC. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Devika G. S, Sudhakar M, Rao J. V. Simultaneous Estimation of Cefixime and Moxifloxacin in Bulk and its Pharmaceutical Dosage form by RP-HPLC. Available from: http://www.orientjchem.org/?p=22728 |
Introduction
Cefixime trihydrate (CEF) is an orally active third generation semi synthetic cephalosporin. Chemically, CEF is (6R,7R)- [(Z)-2-(2-aminothiazol-4-yl)-2-[(carboxymethoxy) imino] acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo [4.2.0]pet-2-ene-2-carboxylic acid trihydrate,[Figure1.a] CEF is official in USP,1 BP,2 and EP.3Moxifloxacin (MOX) (1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo-[3,4-b]pyridin-6-yl]-4-oxo-3-quinoline carboxylic acid hydrochloride) ,[Figure1.b] is new, fourth generation fluoroquinolone with broaden spectrum of antibacterial activity.4-5Moxifloxacin in combination with the 3rd generation cephalosporine cefixime shows great additive and Synergistic effect to treat infectious diseases caused by a Gram-positive or Gram-negative pathogen like Streptococcus pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus.6-7The Innovative tablet combination of Cefixime trihydrate and moxifloxacin was recently approved by DCGI and CDSCO. The present investigation carried out on tablet mixture prepared from commercially available individual tablet dosage form of above mentioned drugs. Literature survey shows that various analytical methods have been reported for estimation of cefixime8-11 and moxifloxacin12-15 individually and combination with other drugs.16-19Only one UV-Visible Spectrophotometric method20 was reported for its simultaneous estimation. HoweverthereisnoRP-HPLCmethodreportedforthesimultaneousestimationof thesedrugsincombineddosageforms. Inthis communication,asimple,precise, reproducibleand accuratereverse-phasehighperformance liquid chromatographic method to estimate cefixime and moxifloxacinintabletdosageformisreported.
 |
Figure.1 Structure of Cefixime trihydrate and Moxifloxacin Click here to View figure |
Material And Methods
Chemicaland Reagents
The gratissamplesofCefixime(CEF)andMoxifloxacin(MOX)wereobtained from Alembiclimited,vadodara.and TorrentPharmaceuticalIndustriesLtd., Ahmedabad.HPLCgradewaterandacetonitrilewerepurchased fromE.Merck(India)Ltd., Mumbai. Potassiumdihydrogen phosphateandorthophosphoricacidof ARGradewere obtained from S.D. Fine Chemicals Ltd., Mumbai.The marketed tablet formulations Suprax®having 400 mg of CEF from Lupin, Mumbaiand Moxif®having 400mg MOX from TorrentPharmaceuticalIndustriesLtd., Ahmedabad were purchased from the local market.
Chromatographicconditions:
Theanalysisofthedrug wascarriedoutona WatersHPLCsystemequippedwitha reversephasePurospherBDSC18 column(250mm×4.6mmid, 5µm particlesize),2695binarypump,a20µl injectionloopanda2487dual absorbancedetectorandrunningonWatersEmpowersoftware.
Standard preparation
Standard stock solution
Standard stock solutions were prepared by dissolving separately 100 mg of each drug in 100 ml of diluent which was a mixture of acetonitrile and phosphate buffer in the ratio of 40:60v/v to get concentration of 1000µg/ml.
Working standard solution
Working standard solutions were prepared by taking dilutions ranging from 20-120µg/ml for both CEF and MOX, respectively.
Validation of the Method
The developed method was validated as per ICH guidelines[21] in terms of specificity, linearity, accuracy, precision, limits of detection (LOD) and quantification (LOQ) and system suitability. The accuracy was expressed in terms of percent recovery of the known amount of the active pharmaceutical ingredient in presence of excipients. The precision (%relative standard deviation, %RSD) was expressed with respect to the intraday and interday variation in the expected drug concentrations. Minor changes in pH of the mobile phase, flow rate, column temperature and detector wavelength were studied to evaluate the robustness of the developed assay method.
Results And Discussion
Optimization of the method
The goal of this study was to develop a single isocratic phase HPLC method for the simultaneous determination of cefixime trihydrate and moxifloxacin.During optimizing the method some important parameters like pH of the mobile phase , concentration of the acid or buffer solution ,percentage and type of the organic modifier,etc.,were tested for a good chromatographic separation. Trials showed that a slightly acidic with reverse phase, aPurospherBDSC18 column gives symmetric and sharp peaks. For this reason,0.01M potassium di hydrogen orthophosphate solution was preferred as an acidic buffer .When triethylamine was used as modifier the method shows a very good resolution between CEF and MOX at pH in the range of six .Finally acetonitrile andpotassium di hydrogen phosphate bufferpH6with intheratioof40:60 v/v was selected as optimal for obtaining well defined and resolved peaks.Thedetectionofthedrugwasmonitored at276nm.Theruntimewassetat10min.Undertheseoptimized chromatographic conditions the retention time obtained for the drugscefixime and moxepril was3.140min and7.001min,respectively.Atypicalchromatogramshowing the separationofthedrugisgivenin[Figure 2]
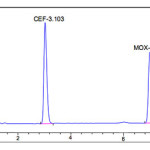 |
Figure 2: Typical chromatogram of Cefixime and Moxifloxacin Click here to View figure |
Method Validation
The developed method was validated as per ICH guidelinesin terms of specificity, linearity, accuracy, precision, limits of detection (LOD) and quantification (LOQ) and system suitability.
System suitability test
The system suitability test performed according to ICH guidelines. The observed RSD values at 1% level of analyte concentration were well within the usually accepted values (≤ 2%). Theoretical plates, tailing factor, resolution between CEF and MOX were determined for both assay and dissolution. The results are all within acceptable limits summarized in [Table1].
Table 1: System Suitability parameters
|
S.NO |
Parameters |
Cefixime |
Moxifloxacin |
Acceptance criteria |
|
1 |
Retention time |
3.12 |
7.04 |
|
|
2 |
RSD of replicate injections |
0.115 |
0.225 |
Not more than 2% |
|
3 |
Asymmetric factor |
1.16 |
0.92 |
Not more than 2 |
|
4 |
Theoretical plates |
6178 |
5210 |
Not less than 3000 |
|
5 |
Resolution factor |
7.41 |
More than 2 |
Specificity
Thespecificityofthemethodwascheckedfortheinterferenceofimpuritiesintheanalysisofa blank solution(withoutany sample)andthena drugsolutionof 20 µg/mLwasinjectedintothecolumn,underoptimized chromatographicconditions, todemonstratetheseparationof bothCEF andMOX from anyoftheimpurities,if present.Astherewasnointerferenceofimpuritiesandalsonochangeintheretentiontime,themethodwasfound to be specificand also confirmed with theresults of analysis of formulation.
Linearity study
The peak areas of CEF and MOX were linear with respect to the concentrations over the range of 20-120µg/mL for both respectively. The slope and intercept value for calibration curve Y =6243.7X—6841.1 (R2= 0.9996) for CEF and 8110.4X—10790(R2= 0.9991) for MOX.The results showed that excellent correlation exists between peak area and concentration of the drugs within the concentration range indicated previously. The data was analyzed by “linear regression least squares fit”, and the parameters are listed in [Table2].
Limit of detection and Limit of quantification
The linearity for CEF and MOX were performed from 20-120µg/ml. Linearity graph was plotted and the correlation coefficient (R2) determined. The limit if detection (LOD) was calculated from the linearity curve using the formula
LOD= 3.3Χ {Residual Standard deviation/Slope}.
The LOD for CEF was confirmed to be 0.5µg/mL and for MOX it was confirmed to be0.82 µg/ml.
The Limit of quantification (LOQ) was calculated from the linearity curve using the formula.
LOQ= 10Χ {Residual Standard deviation/Slope}
The LOQ for CEFwas confirmed to be2µg/mL and forMOX it was confirmed to be2.5 µg/ml.
Table 2: Linearity Study
|
S.NO |
Parameters |
Cefixime |
Moxifloxacin |
|
1 |
Linearity range |
20-120µg/mL |
50-300 µg/mL |
|
2 |
Slope |
6273.7 |
8110.4 |
|
3 |
Intercept |
6841.1 |
10790 |
|
4 |
Correlation coefficient(R2) |
0.9996 |
0.9991 |
|
5 |
Limit of Detection |
0.50 µg/mL |
0.82 µg/mL |
|
6 |
Limit of Quantification |
2µg/mL |
2.5µg/mL |
Precision
Intraday and Interday precision were evaluated by determining the corresponding responses three times on the same day and on 3 different days for CEF and MOX(40,60,80 µg/ml).The results of intra- and inter-day variations are shown in [Table3]. The results obtained from intermediate precision also indicated a good method precision. All the data were within the acceptance criteria.
Table 3: Intraday and interday precision data for the quantitative determination of Cefixime and Moxifloxacin
|
Name of the Drug |
Concentration µg/ml |
Intraday precision |
Interday precision |
||
|
Calculated concentration±SD* |
RSD% |
Calculated concentation±SD* |
RSD% |
||
| CEF |
40 |
39.94±0.413 |
0.41 |
40.14±0.091 |
0.54 |
|
60 |
60.10±0.314 |
0.22 |
59.95±0.143 |
0.22 |
|
|
80 |
81.01±0.415 |
0.81 |
79.96±0.264 |
0.71 |
|
| MOX |
40 |
40.18±0.246 |
0.42 |
40.24±0.126 |
0.62 |
|
60 |
61.29±0.129 |
0.49 |
60.19±0.116 |
0.12 |
|
|
80 |
80.11±0.315 |
0.41 |
79.58±0.744 |
0.84 |
|
*Averageof sixdeterminations
Accuracy
The accuracy of the method was determined by recovery experiments. It wasconfirmedbystudying therecoveryatthreedifferentconcentrations,50%,100%,and 150%ofthose expectedby spiking apreviouslyanalyzedtestsolutionwithadditionaldrug standardsolutions, theanalysisbeing done inreplicate. The %RSDand %relative error in allcases were within the acceptablelimit ( ≤2%). Itis evident fromtheresults ofaccuracy study,reportedin [Table 4]that theproposedmethod enablesveryaccuratequantitativesimultaneousestimationofCEF and MOX
Table 4:Accuracy of the Method
|
Name of the Drug |
Amount (%) of drug added |
Theoretical content (µg/ml) |
Conc.found (µg/ml)±SD* |
Recovery (%) |
RE (%) |
RSD (%) |
|
CEF |
0 |
40 |
39.52±0.222 |
98.8 |
0.54 |
0.562 |
|
50 |
60 |
59.97±0.325 |
99.95 |
0.89 |
0.541 |
|
|
100 |
80 |
80.65±0.564 |
100.81 |
0.11 |
0.699 |
|
|
150 |
100 |
99.97±0.245 |
99.97 |
0.45 |
0.245 |
|
|
MOX |
0 |
40 |
40.46±0.354 |
101.15 |
0.36 |
0.874 |
|
50 |
60 |
59.95±0.324 |
99.91 |
0.76 |
0.540 |
|
|
100 |
80 |
79.93±0.356 |
99.92 |
0.26 |
0.445 |
|
|
150 |
100 |
100.45±0.698 |
100.45 |
0.79 |
0.694 |
*SD= standard deviation(n=3),*RSD=SD/Mean×100,
RE(%)=%Relative Error =(Mean assayed concentration-Added Concentration/ Added Conentration×100)
Robustness and Ruggedness
Robustness studies were carried out after deliberate alterations of flow rate and mobile phase compositions and pH. It was observed that the small changes in these operational parameters did not lead to changes of retention time of the peak interest. The degree of reproducibility of the results has proven that the method is robust and the data are summarized in [Table 5]. The ruggedness of the method was determined by carrying out the experiment on different instrument like Waters HPLC and Shimadzu HPLC and by two different operators using different columns of similar type like Phenomenex C18,HypersilC18 and the results were shown in [Table 6],the low RSD values confirms the ruggedness of the method.
Table5: Statistical data for Ruggedness
|
parameter |
Cefixime |
Moxifloxacin |
||
|
S.D |
%R.S.D |
S.D |
%R.S.D |
|
|
Shimadzu and Waters ( Different Instrument) |
0.312 |
1.256 |
0.741 |
1.245 |
|
Day to day |
0.211 |
1.054 |
0.145 |
1.547 |
|
Analyst to Analyst |
0.145 |
0.984 |
0.19 |
1.121 |
Table 6: Robustness testing of the method
|
Parameter |
Modification |
Cefixime % Recovery |
Moxifloxacin % Recovery |
| pH |
6.2 |
99.63 |
99.54 |
|
6.0 |
99.57 |
99.82 |
|
|
5.8 |
99.92 |
101.06 |
|
| Buffer Composistion(A) |
38 |
99.84 |
100.23 |
|
40 |
101.6 |
100.21 |
|
|
42 |
100.4 |
99.79 |
|
| Flowrate (mL/min) |
0.9 |
101.0 |
99.61 |
|
1.0 |
99.31 |
99.84 |
|
|
1.1 |
101.0 |
99.32 |
Estimation of cefixime and moxepril in tablet dosage form
Twenty tablets of each brand Suprax® and Moxif®were weighed individually and ground to a fine powder. An accurately weighed powder sample equivalent to 100 mg of both CEF and MOX were transferred to 100 ml of volumetric flask anddissolvedin25mL of a 45:60 v/v mixture of acetonitrile and phosphate buffer. The contents of the flask were sonicated for 15 min and a further25mLofthediluentwasadded,theflaskwasshaken continuously for 15 min to ensure complete solubility of the drug. Thevolumewasmadeupwiththediluentandthe solutionwasfilteredthrougha0.45µmembranefilter.This solutionwasfurtherdilutedtogettherequiredconcentrations. Thesolutioncontaining40µg/mlwasinjectedintothe column sixtimes. Theaveragepeakareaofthedrugswascomputed from the chromatograms and the amount of the drug present inthetabletdosageformwascalculatedbyusingthe regression equationobtainedforthepuredrug. Therelevantresultsare furnishedin[Table7].
Table 7: Analysis of formulation
| Drug | Labeled amount(mg) | Amount of mg/tab found* | %Label claim | %RSD |
| Cefixime | 40 | 39.8 | 99.53 | 0.312 |
| Moxifloxacin | 40 | 40.21 | 100.02 | 0.115 |
*Averageof sixdeterminations
Conclusion
The developed method is accurate, simple, economical, rapid and selective for the simultaneous estimation of cefixime and moxifloxacin in bulk and in tablet dosage form without prior separation. The excipients of the commercial sample analyzed did not interfere in the analysis, which proved the specificity of the method for these drugs. The sample preparation is simple,the analysis time is short and the elution is isocratic.Hence, the proposed method can be conveniently adopted for the routine quality control analysis in the combination formulations.
References
- The United States Pharmacopoeia, 29/NF 24. The official compendia of standard Asian Edition. 2007;1654.
- British Pharmacopoeia, British Pharmacopoeia Commission. Vol. 1. London: Her Majesty’s Stationary Office. 2009;397.
- European Pharmacopoeia, Council of Europe. 3rd ed. Strasbourg, France: EDQM Publications.2000;504
- J. A. B. Balfour and L. R. Wiseman, Moxifloxacin,Drugs. 1999;57:363–374.
- BudavariS, eds, In;The MerckIndex.13thed. Merck and Co., Inc; Ruchway USA. 2001;1125.
- Zhanel GG, Ennis K, Vercaigne L, et al. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs. 2002;62:13-59.
- Karlowsky JA, Thornsberry C, Jones ME, Evangelista AT, Critchley IA, Sahm DF. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin Infect Dis 2003;36:963-70.
- Nahata MC. Measurement of Cefixime in serum and cerebrospinal fluid by high performance liquid chromatography. J Liq Chromatogr. 1991;14:3755–60.
- HafizMuhammadArshad,ShahnazGauhar,RaheelaBanoandIyadNaeemMuhammad., Development of HPLC-UVmethodforanalysisofCefiximeinrawmaterialsand in capsule. Jordan Jou of Pharm sci. 2009;2:53-65.
- ShahPB and Pundarikakshudu.K. Spectrophotometric,difference spectros copic andhighperformance liquid chromatographic methods for determination of cefixime in pharmaceutical formulations.American jou of Ana Chem. 2006;89:987-94.
- Khaja Pasha,DrC.S Patil,Dr.K.Vijaykumar,Dr.Sadath Ali andDr.V.B.Chimkod.,Reverse phase HPLC methd for determination of cefixime in Pharmaceutical dosage forms.Research J.Pharma.Bio.Chem.Sci. 2010;3;226-228,
- Motwani SK, Khar RK, Ahmad FJ, Chopra S, Kohli K, et al. Application of a validated stability-indicating densitometric thin-layer chromatographic method to stress degradation studies on moxifloxacin. Anal Chim Acta. 2007;582: 75-82.
- SA Shah, IS Rathod, BN Suhagia, MV Baladaniya An HPTLC method was developed and validated for the estimation of Moxifloxacin in its tablet formulation. Indian J Pharm Sci. 2005;67: 112-115.
- B. B. Ba, R. Etienne, D. Ducint, C. Quentin, and M. C. Saux, Determination of moxifloxacin in growth media by high-performance liquid chromatography.Journal of Chromatography B, 2001;754:107–112.
- A. Laban-Djurdjević, M. Jelikić-Stankov, and P. Djurdjević.Optimization and validation of the direct HPLC method for the determination of moxifloxacin in plasma.Journal of Chromatography B. 2006;844:104–111.
- Madhura V. Dhoka. Vandana T. Gawande, Pranav P. Joshi. Simultaneous estimation of Cefixime Trihydrate and Erdostein in Pharmaceutical dosage form by using RP-HPLC. Int. Jou of Chem Tech Res. 2010;2:79-87.
- M.K Kumudhavalli, Sandeep Sahu, K. Abhiteja, Dr.B. Jayakar. Development and validation of RP-HPLC Method for Simultaneous Determination of cefixime and potassium Clavulanate in tablet dosage form. International Journal of Pharma Recent Research. 2010;2:57-60.
- Ajit R.Wankhede, Prashant Y. Mali, Vikram Karne, Anubha R. Khale, C.S. Magdum. Development and validation of RP-HPLC method for simultaneous estimation of cefixime and cloxacillin in tablet dosage form. Inter Jou of Pharm & Bio Arch. 2010;1: 317-320.
- H. Liang, M. B. Kays, and K. M. Sowinski, Separation of levofloxacin, ciprofloxacin, gatifloxacin, moxifloxacin, trovafloxacin and cinoxacin by high-performance liquid chromatography: application to levofloxacin determination in human plasma,Journal of Chromatography B. 2002;772:53–63.
- RonakK.Patel,RajeshR.Parmar,VishnuMPatel,DushyantA.MethodDevelopmentAndValidationOf Cefixime And MoxifloxacinIn Pharmaceutical Dosage FormBy UV Spectrophotometric Method.Int JouPharmaRes andBio-Sci: 2012;2:81-93.
- ICH,Q2B,Validation of analytical procedures: Methodology, In proceedings of the International Conference on Hormonisation:Geneva,November 1996;1-8.
- S.Ahuja,R.D.Ornaf,M.Dong , U.Neue and A.plasz.Handbook of Pharmaceutical analysis by H PLC. Eds Elsevier, Amsterdam, Netherlands, chapters 2005;6-7;145-216.

This work is licensed under a Creative Commons Attribution 4.0 International License.









