Microwave Assisted Synthesis, Characterization and Antimicrobial Activity of 10H-Phenothiazine Derivatives
G.S. Kalwania*, S. Chomal, S. Choudhary, Radhey Shyam and Sunita Kumari
Department of Chemistry, S. K. Government P.G. College, Sikar - 332 001, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
A new environmental benign method for synthesis of phenothiazine derivatives using microwave irradiation has reported. A mixture of o-substituted aromatic amine and p-substituted phenol converted into biphenyl amine derivative using microwave irradiation. Further, thionation of biphenyl amines under same irradiation has provided the title phenothiazine derivatives. The later compounds then oxidized to their 5, 5-dioxide derivatives by 30 % hydrogen peroxide. All the synthesized title compounds have screened for their antimicrobial activity and their structure confirmed by spectral as well as other analytical data.
KEYWORDS:Phenothiazine; Microwave; Irradiation; Antimicrobial; Spectral
Download this article as:| Copy the following to cite this article: Kalwania G. S, Chomal S, Choudhary S, Shyam R, Kumari S. Microwave Assisted Synthesis, Characterization and Antimicrobial Activity of 10H-Phenothiazine Derivatives. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Kalwania G. S, Chomal S, Choudhary S, Shyam R, Kumari S. Microwave Assisted Synthesis, Characterization and Antimicrobial Activity of 10H-Phenothiazine Derivatives. Available from: http://www.orientjchem.org/?p=22741 |
Introduction
Microwaves based studies have been attracting our attention progressively in the field of organic synthesis. The practical advantages associated with microwave irradiation in organic synthesis have included the enhanced reaction rates, high yields, improved selectivity, reduction of thermal degradation by products(4,14) and environmental friendly reaction conditions(13, 17-18). Moreover, microwave assisted organic synthesis (MAOS) is an acknowledged quick alternative and green technology in the synthetic organic chemistry. The reactions, which are not possible under the conventional conditions can sometime, be carried out by high-energy microwave irradiation.
Thiazine derivatives like phenothiazines and their 5,5-dioxide derivatives have been significantly attracted our interest in medicinal chemistry due to their wide spectrum of pharmacological efficacy(20-34). Phenothiazine derivatives have associated with various pharmacological activities such as antitumor agents (7-11) tranquilizers(15,32), sedative, antiepileptic(27-28), antituberculotic(19), bactericides(5), neuroleptic(21), antimicrobial(1,3,29), antiplatelet(29), analgesic(30) and antidepressive(31). These compounds also exhibit chelating properties towards various metals(2) and have shown the ability to cleave DNA upon photochemical induction(6,12,24,26). A slight change in the substitution pattern of phenothiazine derivatives can cause a remarkable difference in their biological activities. Therefore, efforts made to develop a new environmentally benign method to synthesize phenothiazines and their sulphone derivatives to make them available for beneficial biological importance with minimum undesirable effects. Under present investigation some known phenothiazine derivatives has synthesized by conventionalmethod involving Smiles Rearrangement(9-10). Further, we have made efforts to synthesize these compounds by microwave-assisted operations in varying experimental conditions to optimize the method. Finally, we have developed a new microwave assisted method for synthesis of phenothiazine derivatives.
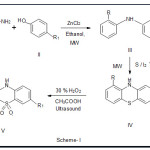
The developed new method has two steps instead of six steps in conventional method involving Smiles rearrangement. In the first step, a biphenyl amino derivative has obtained by exposing a mixture of p-substituted phenol and o-substituted aniline derivatives in minimum quantity of absolute ethanol and ZnCl2 as catalyst to microwave irradiation. The second step has involved the synthesis of phenothiazine via thionation of biphenyl amino derivative under microwave irradiation in presence of iodine (Scheme-I). The phenothiazine derivatives have further oxidized into their new 5, 5-dioxide derivatives by ultrasonic method. All the synthesized phenothiazines have screened for antimicrobial activity. The tri fluoromethyl derivative has shown comparatively better results. All the derivatives have analyzed with their spectral and physico- chemical data and compared to their available literature values(9-10). The physico-chemical data and microbial activities of the synthesized phenothiazines derivatives summarized in table 1to 3.
 |
Scheme 1 Click here to View scheme |
Material and Methods
All chemicals used in the microwave study were of high purity grade (A.R. grade). The melting point of synthesized compounds has not corrected and their purity checked by TLC (thin layer of silica gel) in various non-aqueous solvent systems. Infrared (IR) spectra of all the synthesized compounds recorded using KBr from Shimadzu FTIR, Affinity-1. The NMR spectra has taken by Varian Gemini 400 spectrometer (300MHz) using TMS as an internal standard. The mass spectra have recorded using Jeol SX 102 spectrometer at 70 eV. The reactions carried out in domestic microwave oven. Elemental data obtained from all the synthesized compounds found satisfactory.
Synthesis of Biphenyl Derivatives (III)
An equimolar mixture of o-substituted aniline (I) and p-substituted phenol (II) taken in a flat bottomed flask fitted with a suitable reflux condenser was dissolved in minimum quantity of absolute ethanol and a catalytic amount of zinc chloride added. The reaction mixture so obtained in flask irradiated in microwave oven in intermittently at 30 seconds for 5-6 minutes. After completion of reaction as monitored by TLC, the solid separated out filtered and washed with distilled water. The crude product dried in vacuum and recrystallized from ethanol (Scheme -1).
Synthesis of Substituted Phenothiazines (IV)
A well grinded mixture of biphenyl derivatives (III) (10 mmol), sulphur (20 mmol) and iodine (1% weight of reaction mixture) has been subjected to microwave irradiation intermittently at 30 seconds for 8-10 minutes. The colored solid separated out washed repeatedly with distilled water followed by small amount of alcohol. The crude product was dried and recrystallized from methanol (Scheme -1). These compounds have also been synthesized by conventionally method as described by Gupta et al. (9-10) to compare the physicochemical data. Characterization data of all the synthesized compounds have summarized in table-1.
Synthesis of 5, 5-Dioxide Derivatives of Phenothiazines (Sulphones) (V)
To the 10mmol of the phenothiazine derivative in 15 ml of glacial acetic acid, 5 ml of 30 % hydrogen peroxide added at room temperature with stirring. After complete addition, the contents heated for 15 minutes. When the color of solution changed to yellow, another 5 ml of 30 % hydrogen peroxide added. Then the solution refluxed for one hour in ultrasound bath. After that, the major portion of the solvent evaporated under reduced pressure and the solution poured into beaker containing crushed ice. The yellow residue separated out collected and recrystallized from ethanol to get the desired sulphone derivatives. The physical data have summarized in table 2.
Antimicrobial Activities
All the synthesized phenothiazine derivatives were screened in vitro applying agar plate diffusion technique18 for antibacterial activity against Escherichia coli (Gram negative), and Bacillus subtilis (Gram positive) at both 25 µg/ml, 50 µg/ml concentration. Under same conditions, the Chloramphenicol with 25µg/ml has shown a zone of inhibition 26 mm for gram-negative organisms and Vancomycin with 25µg/ml showed a zone of inhibition 24 mm for gram-positive organism. The antifungal screening of the synthesized phenothiazine derivatives were carried out in vitro by paper disc method against Aspergillus niger and Canadida albicans at 25 µg/ml and 50 µg/ml concentrations by using Griseofulvin at 25 µg/m concentrations as the positive control which has shown zone of inhibition 27 mm and 25.5 mm respectively under same conditions. Antimicrobial activities of synthesized compounds have summarized in table 3.
 |
Table 1: Physico-Chemical Data of Substituted Phenothiazines (IV) |
Spectral studies
The IR spectra of all the synthesized phenothiazine derivatives showed a sharp peak in the region 3325-3395Icm-1 corresponding to the N-H stretching vibrations. Two bands in the region 1530-1535 cm-1 in case of IV-c and IV-e observed which have ascribed to the asymmetric and symmetric valence vibrations of nitro group at position-7. Phenothiazines (IV-e – IV-g) having chloro group at position -1 exhibited a sharp intense peak in the region740-720 cm-1. Spectra of 1-methyl phenothiazines (IV-a to d) have sharp bands in the region 1490-1450 cm-1 and 1380-1375 cm-1 that have assigned to C-H deformation vibrations of CH3 group. Peaks corresponding to deformation vibrations of CF3 in compounds IV-d and IV-g observed in the region 1330-1320 and 1140-1125 cm-1. Sharp band corresponding to carbonyl group in compound IV-b and IV-f has also observed in the region 1690-1685cm-1. We have observed three characteristic peaks in all the phenothiazine sulphone derivatives in the region 1340- 1350 cm-1, 1300-1280 cm-1and 1270- 1240 cm-1. These peaks ascribed to three normal stretching modes of vibrations (V1, V2 and V3) of sulphonyl group in all the sulphone derivatives at position -5.
NMR spectral studies have also extended to synthesized tile compounds. These studies reveal that structures of the compounds are in accordance with their NMR spectra. A singlet peak observed in the region 8.6-8.2 δ ascribed to N-H proton at position-10 in all the phenothiazines. Peaks corresponding to aromatic protons in all compounds and methyl protons in case of compound (IV-a to d) have observed in the region 7.6-7.2 δ and 2.7-2.2 δ respectively. Mass spectral studies of some synthesized compounds have also studied. The fragmentation behavior and molecular ion peaks of the compounds in their mass spectra confirm their structures.
Result and discussion
Generally, phenothiazine derivatives have been synthesizing in six steps through conventional method involving Smiles rearrangement. In first three steps we get 2-aminobenzenethiol from aromatic amines and further three steps are required for synthesis of phenothiazines. Therefore, the conventional method is laborious, lengthy, time consuming and low yield providers with respect to starting material, aromatic amines. Therefore, efforts made to design a new environmentally benign method for synthesis of bioactive title compounds to make available these compounds for pharmacological aspects. Finally, we get a new microwave assisted method for synthesis of substituted phenothiazine derivatives. The reaction yield of final product improved drastically with respect to the initially used amines as compared to the conventional smiles rearrangement and lowered the reaction time.
The developed new method has two steps instead of six step conventional method. In the first step, a biphenyl amino derivative has obtained exposing a mixture of p-substituted phenol and o-substituted aniline derivatives dissolved in minimum quantity of absolute ethanol and ZnCl2 as catalyst under microwave irradiation. The second step has involved the synthesis of phenothiazine via thionation of biphenyl amino derivative under microwave irradiation in presence of iodine as catalyst (Scheme-I). It is observed that the presence of electron withdrawing group at o/p-position of hydroxyl group in phenols favor the overall reaction. The 5, 5-dioxide derivatives of the synthesized phenothiazines have obtained by oxidation with 30 % hydrogen peroxide in glacial acetic acid in ultrasound bath.
Antimicrobial activity against tested microbes for trifluoromethyl derivatives found comparatively better in compared to other derivatives. Antimicrobial results for all the synthesized phenothiazines have summarized in
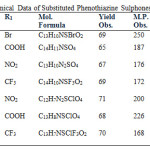 |
Table 2: Physico-Chemical Data of Substituted Phenothiazine Sulphones (V) Click here to View table |
In IR spectra the synthesized compounds showed bands corresponding to secondary amino group at 3325-3395 cm-1 and also for nitro (sym.1530, asym.1340-1350), methyl(defom.1450-1490, 1375-1380), Chloro (720-740), and CF3 (deform.1320-1330,1125-1140) groups. Lowering in N-H absorption frequency in 1-chloro derivatives as compared to 1-methyl derivatives observed which assigned to the weak hydrogen bonding between Cl and hydrogen at position-10 (N-H). All phenothiazine sulphone derivatives have shown three normal stretching modes of vibrations (V1, V2 and V3) of sulphonyl group at position -5. NMR spectra of the synthesized phenothiazines showed well-defined peaks corresponding to N-H, CH3, and aromatic protons in the region 8.2 – 8.6 δ, 2.2 – 2.7 δ and 7.2 – 7.6 δ respectively. Molecular ion peaks and fragmentation pattern in mass spectra confirms the structure of the synthesized compounds.
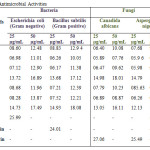 |
Table 3: Antimicrobial Activities |
Conclusion
The conventional method involving Smiles rearrangement for synthesis of title compounds requires chemicals like bromine, chloroform, ammonia solutions etc. in six steps, which are hazardous to health and surrounding environment. The new developed two-step microwave assisted method for synthesis of substituted phenothiazine derivatives is free of hazardous chemicals and proved strategically developed environmentally benign method for synthesis of title compounds in better yield. The antimicrobial activity against tested microbes found comparatively better for trifluoromethyl derivatives. Spectral and analytical data have found in good accordance with their structure.
Acknowledgement
One of the authors, Dr. G. S. Kalwania is thankful to the University Grant Commission (UGC), New Delhi for the financial support. The other authors Mrs. Savita Choudhary is also thankful to UGC for providing financial support through TRF and Radhey Shyam is likewise grateful to ONGC, Mumbai Region for giving the permission to carry out the research and posting him in 14 days on/off pattern at offshore. The authors are also appreciative to colleagues of the department of chemistry for their invaluable suggestions and administration of S.K. Govt. College, Sikar to providing facilities for research work.
References
- Bansode T.N., Shelke J.V. and Dongre V.G., Eur. J. Med. Chem., 44, 5094(2009).
- Barbe, J. and Hurwic, I., Anti. Pharmi. Er., 31(3), 227(1973).
- Bisi A., Meli M., Gobbi S., Rampa A. et al., Bioorg. Med. Chem., 16, 6474(2008).
- Chandak S., Tetrahedron, 51, 10403(1995)
- Dave A.M., Batt K.N., Vandania N.K. and Trivedi P.B., J. Indian Chem. Soc., 65(5), 365(1998
- Decuyper J., Pitte J. et.al, Biochem. Pharmacol., 33, 4025(1984).
- Dumitriu G., Lucescu L., Bicu E. and Belei D., Acta Chemica Iasi, 18, 77(2010).
- Gaina L., Cristea C., Moldovan C., Porumb D. et al., Int. J. Mol. Sci., 8, 70(2007).
- Gupta R.R., Kalwania G.S. and Kumar M., Heterocycles, 16(9), 152(1981).
- Gupta R.R., Kalwania G.S. and Kumar M., J. Heterocyclic Chem., 21, 893(1984).
- Hait et.al, Anticancer Research, 14(59), 1711(1994).
- Intyre R.M. and Gerischer H., Ber. Bunsen Ges. Phys. Chem., 88, 963(1984).
- Kappe C.O., Curr. Opin. Chem. Biol., 6 , 314(2002).
- Kidwa M., Sapra P., Bhushan K.R., Saxena R.K. et al., Montash chem., 113, 85(2000).
- Kulkarni K.G. and Fingerman M., Gen. Pharmacol., 17(60), 67(1986).
- Kumar D., Agarwal R. C., Bhati S. K. and Kumar A., Oriental J. of Chem., 26(2), 497(2010).
- Larhed M., Moberg C. and Hallberg A., Acc. Chem. Res., 35, 717(2002).
- Lidstrom P., Tirrey J., Wathey B. and Westman J., Tetradedron, 57, 9225(2001).
- Madrid P.B., Polgar W.E., Tolla L. et al., Bioorg. Med. Chem. Letters, 17, 3014(2007).
- Majumder A., Sen D., Das J. and Ghosh P., J. Pharma. Sc. Inn., 1(2), 41(2012).
- Makareeva E.N., Lozovskaya E.L. and Sapezhinskii I.I, Biofizika, 43(2), 18(1998).
- Marszalek M., Nagane S., Ichake A., Baker R. H. et al., J. Mater. Chem., 22, 889(2012).
- Motohashi N. et.al, Cancer Invest., 9(3), 305(1991).
- Motohashi N., Anticancer Research, 11, 1125(1991).
- Motten A.G., Buettner G.R. and Chignell C.F., Photochem. Phobiol., 42, 9 (1985).
- Nvati et.al, Anticancer Drugs, 6(5), 693(1996).
- Nishiwaki E., Nkagawa H., Jakasaki M. et.al, M. Heterocycles, 31, 1763(1990).
- Senova Z.P., and Byull, Eksp. Bio. Med., 83(3), 298(1977).
- Shah J.J. and Tenn J., Acad. Sci., 52(1), 19(1977).
- Silva G.A., Costa L.M.M., Brito F.C.F., Miranda A.L.P. et al., Bioorg. Med. Chem., 12, 3149(2004).
- Singh P., Peshin P.K. and Krishnamuthy D., Indian Vet. J., 71(10), 98(1994).
- Sparatore A. and Sparatore F., Farmaco., 49(1), 5(1994).
- Toama M.A., Elfatatry H.M. and Folaha B.F., J. Pharm. Sci., 67(1), 23(1978).
- Yin J.F., Chen J. G., Lin J. T., Bhattacharya D. et al., J. Mater. Chem., 22, 130(2012).
- Yun H. D., Yoo H. S., Park Y. S. and Woo J. W., Advanced Materials Research, 418, 153(2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









