Microwave Assisted Synthesis and Spectral Analysis of Schiff Bases Derived from 2-Amino-5-Aryl-1,3,4-Oxadiazoles
Sanjeev Kumar1,, Sneha Yadav1,, Sudha Jadon1, Vipin Kumar1, Abdalla M. Khedr2 and Kishan C. Gupta*
1Department of Chemistry, Agra College, Agra - 282 002, India. 2Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt. Anand College of Pharmacy, Keetham, Agra - Mathura Road, Agra - 282 007, India
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Microwave assisted synthesis of new Schiff bases derived from 2-amino-5-substituted aryl-1,3,4-oxadiazoles with substituted aromatic aldehyde have been carried out. The chemical structures of the prepared Schiff bases have been investigated by analytical and spectral methods.
KEYWORDS:Microwave assisted synthesis; Schiff bases; Spectral analysis
Download this article as:| Copy the following to cite this article: Kumar S, Yadav S, Jadon S, Kumar V, Khedr A. M, Gupta K. C. Microwave Assisted Synthesis and Spectral Analysis of Schiff Bases Derived from 2-Amino-5-Aryl-1,3,4-Oxadiazoles. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Kumar S, Yadav S, Jadon S, Kumar V, Khedr A. M, Gupta K. C. Microwave Assisted Synthesis and Spectral Analysis of Schiff Bases Derived from 2-Amino-5-Aryl-1,3,4-Oxadiazoles. Available from: http://www.orientjchem.org/?p=22785 |
Introduction
Schiff bases or azomethines are an important class of compounds reported to possess carbon nitrogen double bond which is responsible for various biological activities1 including antibacterial2, antifungal3, anti-inflammatory4, antipyretic5, antitumor6,7, anticancer8 activities. They are also used as starting material for the synthesis of industrial9 and biologically active β-lactum10. Further Schiff bases have also been reported to possess chelatogenic properties, so form ligands in coordination chemistry11,12. Various 1,3,4-oxadiazoles have been reported to possess analgesic13, antihypertensive14, antifungal15, anti-inflammatory16 and anticancer17 activities.
Taking the above facts into consideration and our interest in the synthesis of biologically active heterocyclic, it was thought to synthesize Schiff bases of 2-amino-5-aryl-1,3,4-oxadiazoles by condensation with substituted aryl aldehydes.
Experimental
Materials and Methods
Melting points of newly synthesized compounds were determined in open capillary method using Tempo make melting point apparatus and are uncorrected. The purity of the compounds were checked by TLC (silica gel G) using benzene:chloroform:methanol (5:4:1) solvent system. IR spectra were measured on a Perkin-Elmer IR spectrophotometer as KBr discs (4000–200 cm-1) at the Micro-analytical unit of Tanta University, Egypt. The 1H-NMR spectrum were recorded on a Bruker DMX 750 (500 MHz) using d6 DMSO as solvent and TMS as internal standard. The UV-Vis spectra were recorded on a Cary-400 double beam recording spectrophotometer within the wavelength range 190-700 nm. Electron spray ionization (ESI) mass spectra were carried out using a Shimadzu LCMS-2010 eV mass spectrometer at the Gakushuin University (Japan).
Preparation of Schiff Base Derivatives
Classical Method
The Schiff bases of 2-amino-5-substituted aryl-1,3,4-oxadiazole were prepared by refluxing equimolar quantity of oxadiazole (0.01mole) and substituted aryl aldehyde (0.01mole) in 25 ml of ethanol in round bottom flask for 4–6 hr. The volume of alcohol was reduced to half under reduced pressure. The resulting concentrated reaction mixture was added to ice cold water, Schiff base precipitated out, filtered off, washed with water, dried and purified by recrystallization from alcohol.
Microwave Assisted Method
Equimolar quantities of substituted aryl aldehyde (0.01 mole) and 0.01 mole of 2-amino-5-aryl-1,3,4-oxadiazole were dissolved in 25 ml of ethanol and taken in conical flask fitted with guard tube. The reaction mixture was subjected to microwave irradiation (MW). The heating was continuing for 2-4 min, thin layer chromatography (TLC) of the reaction mixture was carried out after every half minute to check completion of the reaction. When the reaction was complete, it was allowed to cool at room temperature and poured into crushed ice in a beaker, the precipitated product was filtered off, washed with water, dried and recrystallised from appropriate solvent.
Results and Discussion
2-Amino-5-substituted aryl-1,3,4-oxadiazole Schiff bases (SB1-SB7) were synthesized according to Scheme-1 by both classical method and microwave-assisted methods (Table-1).
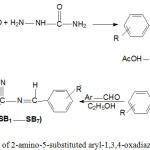 |
Scheme 1: Synthesis of 2-amino-5-substituted aryl-1,3,4-oxadiazole Schiff bases. |
The data listed in Table-1, shows that there was significant difference in time taking for the preparation of Schiff bases by conventional method which ranges from 5-6 hours. While the preparation of all the Schiff bases using microwave method takes only 3-4 minutes for completion of reaction. Similarly there was significant difference in yield of the Schiff bases prepared by conventional and microwave-assisted synthesis. Conventional method gave poor yield of Schiff bases ranging from 62.3-85.0%, while microwave method provide better yield ranging between 70.2-96.0%. This clearly indicates that preparation of Schiff bases by microwave method takes much less time; provide better yield and cleaner product as compared to conventional method. Thus household microwave can be used to carry out the organic synthesis.
Table 1: Physical characterization of synthesized Schiff bases (SB1-SB7).
|
Comp. no. |
R |
R׳ |
Molecular formula |
Molecular weight |
M.P. (oC) |
Classical method |
Microwave method |
||
|
Time (hrs) |
Yield (%) |
Time (min) |
Yield (%) |
||||||
|
SB1 |
H |
H |
C15H11N3O |
249.27 |
235 |
5 |
65.7 |
3 |
92.1 |
|
SB2 |
H |
4-NO2 |
C15H10N4O3 |
294.27 |
145 |
6 |
74.9 |
3 |
82.1 |
|
SB3 |
3-Br |
3-Br |
C15H9N3Br2O |
407.06 |
200 |
6 |
65.0 |
4 |
72.1 |
|
SB4 |
3-Cl |
3-Cl |
C15H9N3Cl2O |
318.16 |
220 |
6 |
62.3 |
4 |
70.2 |
|
SB5 |
4-F |
4-OH |
C15H10N3O2F |
283.26 |
120 |
5 |
62.4 |
3 |
77.0 |
|
SB6 |
3-NO2 |
3-NO2 |
C15H9N5O3 |
339.27 |
160 |
6 |
65.8 |
4 |
71.0 |
|
SB7 |
4-OCH3 |
4-NO2 |
C16H12N4O4 |
342.30 |
190 |
5 |
85.0 |
4 |
96.0 |
The structures of the synthesized Schiff bases (SB1-SB7) were elucidated on the basis of IR, 1H-NMR, UV-Vis and mass spectral data which are given in Table-2. IR spectra clearly showed a strong n(C=N) at 1598-1535 cm-1 and n(C-O-C) absorption band around 1110-1056 cm-1. The stretching vibration of aromatic –CH was observed within 3166-3062 cm-1 range. Azomethine hydrogens of Schiff bases showed three absorption band at 2986-2874 cm-1 [n(N=C-H)], 1286-1228 cm-1 [d(N=C-H)] and 1056-1019cm-1 [g(N=C-H)]. These IR data confirmed the presence of specific functional groups present in the final products.
1H-NMR spectra exhibited a multiplet at 6.28-8.39 ppm for hydrogens of the aromatic rings. The azomethine proton (-CH=N-) appeared as a singlet at 8.68-10.45 ppm. The spectra of SB5 showed a singlet at 10.61 ppm for –OH group, while SB7 showed a singlet for –OCH3 group at 3.32 ppm.
The electronic absorption spectra of the synthesized Schiff bases were scanned in methanol within 200-500 nm and showed two main bands. The shorter wavelength band appeared at 204-214 nm range is attributed to the high energy p—p* transition corresponding to the 1La—1A state in the aromatic moiety18. The longer wavelength band located at 267-283 nm range due to n—p* transition within the CH=N group19.
Table 2: Spectral data of synthesized compounds (SB1-SB7).
|
Comp. no. |
UV-Vis (λmax, nm) |
IR (KBr cm-1) |
1H-NMR (DMSO-d6, δ ppm) |
|
SB1 |
207, 277 |
1591 (C=N), 1069 (C-O-C), 3113 (Ar–CH), 2978, 1248, 1027 (-N=C-H-). |
7.24-7.99, 10.23 |
|
SB2 |
211, 272 |
1597 (C=N), 1056 (C-O-C), 3114 (Ar–CH), 2978, 1239, 1056 (-N=C-H-). |
6.47-7.98, 10.24 |
|
SB3 |
207, 281 |
1597 (C=N), 1106 (C-O-C), 3106 (Ar–CH), 2950, 1255, 1042 (-N=C-H-). |
7.31-7.87, 10.01 |
|
SB4 |
204, 283 |
1596 (C=N), 1110 (C-O-C), 3109 (Ar–CH), 2970, 1257, 1046 (-N=C-H-). |
7.32-7.89, 8.68 |
|
SB5 |
206, 276 |
1597 (C=N), 1075 (C-O-C), 3166 (Ar-CH), 2874, 1286, 1034 (-N=C-H-). |
6.28-8.04, 9.77, 10.61 (s, 1H,-OH) |
|
SB6
|
211, 267 |
1598 (C=N), 1092 (C-O-C), 3062 (Ar-CH), 2986, 1228, 1030 (-N=C-H-). |
6.50-7.98, 10.45 |
|
SB7 |
214, 273 |
1535 (C=N), 1079 (C-O-C), 3129 (Ar-CH), 2889, 1256, 1019 (-N=C-H-). |
7.03-8.39, 10.13, 3.32 (s, 3H,-OCH3) |
Under EI condition, the mass spectra of the Schiff bases showed the prominent mass peaks at m/z 249.3, 407.1, 318.2, 339.3 and 323.1 corresponding to the molecular weight of the parent ion [M] + of SB1, SB3, SB4, SB6 and SB7 respectively. SB2 and SB5 showed the prominent mass peaks at m/z 295.3 and 284.3 due to M++1. All the obtained spectral and analytical data confirm the structure and purity of the synthesized Schiff bases (SB1-SB7).
Conclusion
From above data it can be concluded that microwave assisted synthesis is ideal for synthesis of Schiff bases because it provide a facile inexpensive, simple and fast method for synthesis of Schiff bases as compare to reported conventional method in reducing the heating time as well as improving the yield of the clean product.
Acknowledgments
We gratefully acknowledge our sincere thanks to Dr. Mohamed El-Zarie (Gakushuin University, Japan) for his support of this work by providing the 1H-NMR and mass spectral analysis.
References
- Dhar D.N. and Taploo C.L., J. Sci. Ind. Res., 41, 501 (1982).
- Hou N., Xu L.J. and Pao Y.H., Medline., 27, 732 (1992).
- Pandeya S.N., Ram D.S., Nath G. and Cleri E.D., Arzneimittel Forschung., 50, 55 (2000).
- Pavlov H., Litina D.J. and Geronikaki A.A., Drug Des. Discov., 15, 199 (1997).
- Jayasukhalal R., Merchant and Chothia D.S., J. Med. Chem., 40, 194 (1970).
- Deliwala C., J. Med. Chem., 49, 450 (1971).
- Dash B., Patra M. and Paraharaj S., Indian J. Med. Chem., 19B, 894 (1980).
- Holla B.S., Veerendra B., Shivananda M.K. and Poojary B., Eur. J. Med. Chem., 38(7), 759 (2003).
- Aydogan F., Ocal N., Turgut Z. and Yolacan C., Bull. Korean Chem. Soc., 22, 47 (2001).
- Taggi A.E., Hafez A.M., Wack H., Young B., Ferraris D. and Lectka T., J. Am. Chem. Soc., 124, 6626 (2002).
- Arora K. and Sharma K.P, Synth. React. Inorg. Met. Org. Chem., 32, 913 (2003).
- Vigato P.A. and Tamburini S., Coord. Chem. Rev., 248, 1717 (2004).
- Ram V.J., Indian J. Chem., 27B, 825 (1998).
- Ponticello G.S., Engelhardt E.L. and Baldwin J.J., Indian J. Hetrocycl. Chem., 17, 425 (1980).
- Sahin G., Palaska E., Ekizoglu M. and Ozalp M., Farmacia., 57, 539 (2002).
- Ramalingam T., Deshmukh A.A., Sattur P.B., Sheth U.K. and Naik S.R., J. Indian Chem. Soc., 26, 58 (1981).
- Holla B.S., Prasnna C.S., Poojary B., Rao K.S., Shriava, Bhatt U. and Ganesh, Indian J. Chem., 43B, 864 (2003).
- Issa R.M., Khedr A.M. and Rizk H., J. Chin. Chem. Soc., 55, 875 (2008).
- Moustafa M.E., El-Mossalamy E.H. and Amin A.S., Monatsh. Chem., 126, 901 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









