Effects of MWNTs on Flame Retardation and Thermal Stabilization Performance of Phosphorus-containing Flame Retardants in Polypropylene
Mohammad Shahvazian* and Mohammad Reza Seyedmir
Faculty of textile engineering, Yazd Branch, Islamic Azad University, Yazd, Iran.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Effects of multi-walled carbon nanotubes (MWNTs) on flame retardation as well as thermal stabilization efficiency of two phosphorus-containing flame retardant systems i.e. ammonium polyphosphate/pentaerythritol (APP/PER) and red phosphorus (RP) in polypropylene (PP) have been investigated. Limiting oxygen index (LOI), thermo-gravimetric analysis (TGA), melt flow index (MFI) and tensile tests have been performed in this study. It was shown that the addition of MWNTs alone at a minimum loading level of 4 wt% improves thermal stability of PP considerably without any undesirable effect on its flow-ability and mechanical properties. Furthermore, addition of MWNTs alone resulted in a slight improvement of flammability of the polymer. However, comparison between thermal stability and flame retardancy of PP samples containing a combination of MWNTs and APP/PER or RP with those of the samples containing APP/PER or RP alone proved that MWNTs interfere with thermal stabilization and flame retardation efficiency of both APP/PER and RP in the polymer.
KEYWORDS:Polypropylene; Ammonium polyphosphate/pentaerythritol; Red Phosphorus; Multi-walled carbon nanotube
Download this article as:| Copy the following to cite this article: Shahvazian M, Seyedmir M. R. Effects of MWNTs on Flame Retardation and Thermal Stabilization Performance of Phosphorus-containing Flame Retardants in Polypropylene. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Shahvazian M, Seyedmir M. R. Effects of MWNTs on Flame Retardation and Thermal Stabilization Performance of Phosphorus-containing Flame Retardants in Polypropylene. Available from: http://www.orientjchem.org/?p=22669 |
Introduction
Polypropylene (PP) is one of the most widely used polyolefins and has a broad range of applications such as automotive parts, cables, electronics and architectural materials and so on. However, combustibility and melt dripping during its burning are two disadvantages for this commodity polymer. So, it is necessary to incorporate some flame retardant into PP for the applications where the product is subjected to the fire hazard. Among commercial flame retardants used for PP, halogen containing flame retardants have a pronounced flame retardation activity. However, they produce large amounts of smoke and corrosive and irritating gases on burning and thus their use in some applications is being restricted [1-2]. It is therefore worthwhile to investigate on halogen-free flame retardation of PP. In recent years, intumescent flame retardants (IFR) are introduced as a new generation of flame retardants for polypropylene with less smoke, toxicity and corrosion as well as lack of any molten dropping [3-4]. Unfortunately, high levels of loading of flame retardant are generally needed to achieve a reasonable flame retardancy which contributes to unacceptable changes in physical and mechanical properties of polypropylene composites. As a result of this fact, nanoparticle materials have attracted an interest for their ability to improve the mechanical and thermal properties [5]. Moreover, acceptable flammability properties of polymers can be achieved by polymer/carbon nanotube nanocomposites [6]. However, the question that is remained unanswered is that if there is any synergistic effect in flame retardation activities in PP between the traditional phosphorus-containing flame retardants and carbon nanotubes. Therefore, the present work was devoted to investigation on the effects of multi-walled carbon nanotube on thermal stability and flame retardancy of PP composites containing two different phosphorus-containing flame retardant systems including ammonium polyphosphate (APP)/pentaerythritol (PER) and red phosphorus (RP)with different levels of loading have been studied.
Experimental part
Materials
The matrix polymer used in this study was PP supplied by BIPC (Poliran PI0800, melt mass flow rate of 10 gr/10 min at 230°C). Ammonium polyphosphate (APP) was purchased from Clariant and pentaerythritol (PER) and red phosphorus was obtained from merck KGaA. MWNTs were supplied by RIPI (Iran).
Sample preparation
Composite samples were prepared by melt blending of the polypropylene with a certain amount of the other materials specified by their own formulation (Tab. 1) in a Brabender internal mixer with a rotor rate of 60 rpm within 15 minutes. The mixer temperature was set at 190 °C [7]. Polypropylene melted in about 3 min. and the fillers were added at this time and mixing was continued for 12 min. All samples for measuring flammability properties (20 ×10×0.2 mm thickness) were compression molded at 190 °C under a pressure of 25 bar for a duration of 15 min.
Table :1 Formulation of PP samples used for the study.
| Sample designation | Composition (wt %) |
| Blank | PP (100%) |
| C2 | PP (98%) + CNT (2%) |
| C4 | PP (96%) + CNT (4%) |
| C6 | PP (94%) + CNT (6%) |
| AP10 | PP (83%) + APP (10%) + PER (7%) |
| AP15 | PP (78%) + APP (15%) + PER (7%) |
| AP20 | PP (73%) + APP (20%) + PER (7%) |
| RP2.5 | PP (97.5%) + RP (2.5%) |
| RP5 | PP (95%) + RP (5%) |
| RP10 | PP (90%) + RP (10%) |
| APC2 | PP (76%) + APP (15%) + PER (7%) + CNT (2%) |
| APC4 | PP (74%) + APP (15%) + PER (7%) + CNT (4%) |
| RPC2 | PP (93%) + RP (5%) +CNT (2%) |
| RPC4 | PP (91%) +RP (5%) + CNT (4%) |
Thermal stability and flammability measurements
Thermogravimetric analysis was conducted in a nitrogen atmosphere from room temperature to 500○C at a heating rate of 10 °C/min. Temperatures corresponding to 5% , 10%, 20% and 50% weight loss due to thermal decomposition were determined.
Flammability of the samples was evaluated by measuring their Limiting Oxygen Index (LOI) according to ASTM D 2863
Rheological and mechanical properties measurements
Melt flow index of the samples was measured according to ASTM D1238 at 230°C/2.16 kg. Tensile properties of samples were determined according to ASTM D638 using an Instron 4411 instrument.
Results and discussion
Flammability measurements
The flammability was characterized by LOI measurements. Tab. 2 shows the LOI values and dripping behavior of all the specimens. PP ignites easily and its LOI is only 17.0 with heavily dripping. As it can be clearly seen in Tab. 2, the APP/PER system effectively improves flame retardancy of PP so that its LOI reaches 24 when the amount of APP loading is 10 wt % and its LOI becomes 30 with 20 wt %. Moreover, there is no dripping for them. It is obvious that there is no significant change among the LOI values of the samples with different levels of red phosphorus concentration. The LOI values of carbon nanotube containing samples, i.e. C2, C4 and C6 are about 18. It shows that the loading of carbon nanotubes improves LOI value of PP slightly.
Table :2 Results of LOI measurements on PP samples.
| Sample designation |
LOI value |
Drip |
| Blank |
17 |
Heavily |
| C2 |
19 |
Some |
| C4 |
19 |
Some |
| C6 |
18 |
Some |
| AP10 |
24 |
No |
| AP15 |
28 |
No |
| AP20 |
30 |
No |
| RP2.5 |
20 |
Little |
| RP5 |
21 |
Little |
| RP10 |
21 |
Little |
| APC2 |
26 |
No |
| APC4 |
22 |
No |
| RPC2 |
20.5 |
No |
| RPC4 |
20.5 |
No |
In order to evaluate effects of MWNTs on flame retardation efficiency of APP/PER and RP, PP samples containing both MWNTs and APP/PER or RP were prepared. Amounts of APP/PER and RP loading in these samples which are presented in Tab. 1 were chosen according to the results of LOI measurements. As it is seen in Tab. 2, the presence of MWNTs decreases values of LOI of the samples containing APP/PER but the samples still show reasonable flame retardancy. On the other hand, MWNTs has no effect on flame retardation efficiency of RP so that the values of LOI for the samples containing both MWNTs and RP and the samples containing only RP at the same level are almost the same [8].
Besides, by comparing the LOI values of samples containing RP alone and those containing APP/PER at the same level of loading, it could be concluded that the halogen-free flame retardant APP/PER is a more effective flame retardant for PP than RP [9].
Thermal degradation studies
Thermal stability of the samples and the amount of their decomposition at varying temperatures are analyzed by TGA. Figs. 1 and 2 show the TGA curves for the first group of the samples (those containing a single flame retardant system) and the second group (those containing a combined flame retardant system), respectively, by analyzing them in nitrogen atmosphere. Initial decomposition temperature (IDT) is defined as the 5% weight loss in TGA experiments [9]. The IDT values as well as values of temperatures corresponding to 10 (T10), 20 (T20) and 50% weight loss (T50) for the PP samples determined from the TGA curves are presented in Tab. 3. It can be inferred from the results presented in Tab. 3 that the addition of 2% by weight of MWNTs not only has no beneficial effect on PP but also it slightly decreases IDT of PP. However, C4 sample has the highest IDT among the specimens with an amount of 365.4°C [8]. Moreover, the temperatures corresponding to 10, 20 and 50% decomposition for the samples containing (MWNTs) get higher values than those for other samples. Among the other samples, the greatest values for measured decomposition temperature belong to the samples containing only RP as flame retardant.
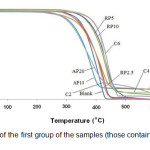 |
Figure :1 TGA curves of the first group of the samples (those containing only one flame retardant system). Click here to View figure |
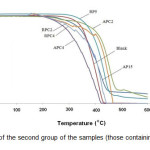 |
Figure : 2 TGA curves of the second group of the samples (those containing a combined flame retardant system). Click here to View figure |
In addition, it can be inferred from the data presented in Tab. 3 that IDT values of the samples containing APP/PER are lower than that of the blank sample. On the other hand, the samples containing RP have the best thermal stability and it seems that the optimum level of RP loading in this regard is 5% because no significant change in IDT value is observed by increasing the amount of RP loading from 5% to 10%.
Table :3 Results of thermal degradation studies on PP samples.
| Sample designation |
IDT (°C) |
T10 (°C) |
T20 (°C) |
T50 (°C) |
| Blank |
320 |
340.5 |
359.7 |
390.6 |
| C2 |
310.8 |
334.1 |
359.9 |
399.6 |
| C4 |
365.4 |
384.5 |
405.3 |
429.2 |
| C6 |
360.4 |
383.9 |
404.3 |
432.5 |
| AP10 |
315.3 |
342.1 |
367.7 |
409.1 |
| AP15 |
282.1 |
320 |
352.9 |
400.1 |
| AP20 |
272.6 |
297.6 |
323.9 |
372.8 |
| RP2.5 |
310.7 |
330.6 |
356.3 |
399 |
| RP5 |
345.1 |
363.3 |
385.3 |
420.5 |
| RP10 |
346.4 |
367.5 |
390.9 |
426.5 |
| APC2 |
287.4 C |
361.5 |
406.9 |
446.5 |
| APC4 |
240.5 |
269.1 |
304.2 |
361.5 |
| RPC2 |
250.4 |
372.7 |
381.8 |
437.4 |
| RPC4 |
246.5 |
289.5 |
338.6 |
404.4 |
Meanwhile, slope of the TGA curves in Fig. 1 which indicates the rate of the weight loss or interchangeably, rate of thermal decomposition of the samples is less for the samples containing APP/PER in comparison with the other samples specially those containing MWNTs. As it is seen in Fig. 1, thermal degradation occurs between 272.6 °C and 500 °C and the observed trend of weight loss is the same for all the samples. Moreover, no decomposition occurs beyond 500 °C for all specimens. The behavior has been already observed for IFR flame retarded samples and could be attributed to the formation of a phosphorus or carbon layer on the surface of the samples [6].
Besides, as it is seen in Fig. 2, all of the samples containing both APP/PER or RP and MWNTs has decomposes sooner than the Blank sample but the degradation in the samples are slower and the final temperature of decomposition is more than the Blank sample. Furthermore, the trend of weight loss for the samples containing both APP/PER or RP and MWNTs is not similar to those for the corresponding samples without MWNTs. Some non-uniformity is seen in the curves which might be assigned to unknown thermo-chemical reactions occur in these samples at elevated temperatures. These probable thermo-chemical reactions could be regarded as a reason for the reduced thermal stability of the samples containing a combined flame retardant system.
Furthermore, as it could be inferred from the results given in Tab. 3, IDT values for APC2 and AP15 samples which contain the same amount of APP/PER are almost the same indicating that the addition of MWNTs at this level of loading has no positive effect on thermal stability of AP15 sample. Nonetheless, T20 and T50 values for APC2 sample are more than 40 °C higher than those of AP15 sample which indicates that the addition of MWNTs lowers the rate of thermal degradation of AP15 sample. As the amount of improvement in T20 and T50 values of PP (the Blank sample) through the addition of both APP/PER and MWNTs is higher than the sum of the individual improvement in those values, respectively, by the addition of APP/PER and MWNTs, it could be assumed that there is a synergistic effect between APP/PER and MWNTs in this regard. Meanwhile, regarding T20 and T50 values for all samples, APC2 sample shows the best result which is a noticeable achievement. However, by comparing IDT, T10,T20 and T50 values for APC4 and AP15 samples it could be concluded that thermal stability of AP15 not only does not increase in the presence of 4 wt% of MWNTs but also faces a noticeable decrease so that IDT, T10,T20 and T50 values of APC4 sample are about 40-50 °C lower than those of AP15.
On the other hand, comparing IDT, T10,T20 and T50 values for RPC2 and RPC4 samples with those of RP5 indicates that MWNTs in both levels of loading has a negative effect on thermal stability of RP5 sample. Furthermore, the diminishing effect of MWNTs on thermal stability of RP5 sample is more pronounced at the higher level of MWNTs loading.
Melt flow index measurements
Melt flow index (MFI) values for all the samples are shown in Fig. 3. High value of MFI means that the material is easy to flow and is thereby easy to process. Quite often low MFI values create form-filling problems and are therefore not desirable from the processing point of view. By comparing the MFI values of the Blank sample with the samples containing only one flame retardant system it could be inferred that addition of both of the APP/PER and RP flame retardant systems increases MFI of the PP and the amount of increase is more pronounced in the presence of APP/PER system. Thus, APP/PER and RP not only has no undesirable effect on flow-ability of PP but also improves it. On the other hand, MFI value of the C2 sample is slightly higher than that of the Blank sample but increasing the amount of MWNTs loading leads to a decrease in MFI value so that MFI value of the C4 and C6 samples are slightly lower than that of the Blank sample. Melt viscosity increase by addition of carbon nanotube has been observed by others and is attributed to the formation of a continuous MWNTs network structure in the polymer [10]. Furthermore, by comparing MFI values of APC2, APC4 and AP15 samples it could be inferred that the addition of MWNTs to AP15 sample leads to a decrease in MFI value which is proportional to the amount of value of MWNTs loading. However, the MFI values of APC2 and APC4 are still higher than that of the Blank sample. Comparison between MFI values of RPC2 and RPC4 samples with that of RP5 again leads to the deduction that the melt viscosity of the filled PP sample is increased by addition of MWNTs.
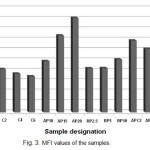 |
Figure :3 MFI values of the samples. Click here to View figure |
Mechanical properties measurements
Tensile strength of the samples is presented in Fig. 4. Comparison between the tensile strength of the Blank sample and that of the samples containing only APP/PER flame retardant system reveals that as it is expected the addition of the APP/PER causes a decline in tensile strength of PP which increases with increasing the amount of APP/PER loading in the sample [11]. On the other hand, one of the attractive features of RP is its negligible effect on mechanical properties of the polymer [12]. As it is seen in Fig. 4, addition of the RP not only doesn’t decrease tensile strength of PP but also causes a slight increase in its value which is more at lower levels of RP loading. Meanwhile, tensile strength of the C2 sample is slightly lower than that of the Blank sample but increasing the amount of MWNTs loading leads to an improvement in tensile strength so that tensile strength of the C6 sample is almost the same as that of the Blank sample. Furthermore, by comparing tensile strength of APC2 and APC4 samples with that of AP15 samples one can conclude that the addition of MWNTs to AP15 sample has no significant effect on tensile strength of AP15 sample. Thus, comparison between tensile strength RPC2 and RPC4 samples with that of RP5 samples proves that tensile strength of RP5 sample is not influenced by the addition of MWNTs.
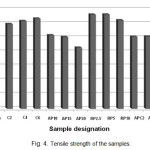 |
Figure : 4 Tensile strength of the samples. Click here to View figure |
Conclusion
It was proved that MWNTs when used at an optimum level of 4 wt% improve thermal stability and flammability of PP without any undesirable effect on its flow-ability and mechanical properties. Although, the efficiency of MWNTs in flame retardation of the polymer is significantly less than that of the both flame retardant systems, ammonium polyphosphate /pentaerythritol and red phosphorus, but MWNTs could stabilizes the polymer against thermal degradation more effective than the both flame retardant systems. However, MWNTs interfere with thermal stabilization and flame retardation efficiency of both APP/PER and RP flame retardant systems in the polymer.
Acknowledgments
This paper has been extracted from a research project .The investigators are thankful to Islamic Azad University, Yazd branch for the financial support of this project.
References
- Chiang, W. Y.; Hu, H. C. J. Appl. Polym. Sci. 2001 82, 2399.
- Lu, S. Y.; Hamerton, I. Prog. Polym. Sci. 2002, 27, 1661.
- Li, Q.; Zhong, H.; Wei, P.; Jiang, P. J. Appl. Polym. Sci. 2005, 98, 2487.
- Paul, D. R.; Robeson, L. M. Polymer, 2008, 49, 3187.
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J. M.; Dubois, Ph. Mater. Sci. Eng. R. Rep. 2009, 63, 100.
- Cipiriano, B.; Kashiwagi, T.; Raghavan, S. R.; Yang Y.; Grulke, E. A.; Yamamoto, K.; Shields, J. R.; Douglas, J. F. Polymer 2007, 48, 6086.
- Chen, Y.; Wang, Q. Polym. Degrad. Stab. 2007, 92, 280.
- Li, Q.; Jiang, P.; Su, Z.; Wei, P.; Wang, G.; Tang, X. J. Appl. Polym. Sci. 2005, 96, 854.
- Zhang, Q.; Xing, H.; Sun, C.; Xiang, H.; Jiang, D.; Qin, L. J. Appl. Polym. Sci. 2010, 115, 2170.
- Rizvi, R.; Khan, O.; Naguib, H. E. Polym. Eng. Sci. 2011, 51, 43.
- Stark, N. M.; White, R. H.; Mueller, S. A.; Osswald, T. A. Polym. Degrad. Stab. 2010, 95, 1903.
- Zhang, S.; Horrocks, R. Prog. Polym. Sci. 2003, 28, 1517.

This work is licensed under a Creative Commons Attribution 4.0 International License.









