Effect of Chemical Parameters on the Properties of Hydrogels Prepared by using Gamma Radiation Polymerization
Mohammad Sadeghi*, Fatemeh Shafiei and Esmat Mohammadinasab
Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak, Iran
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Several hydrogels were prepared using radiolytic polymerization of aqueous solutions of 2-acrylamido-2-methylpropanesulfonic acid containing appropriate comonomer such as acrylic acid and collagen. The hydrogels have been prepared at an irradiation dose of 25 kGy. The effects of the chemical structure of the monomer(s) and crosslinking agent on the yield of homopolymer(s) or copolymers and reaction time have been studied. This crosslinking agent include N, N’-methylene bisallyamide (MBA).
KEYWORDS:Collagen; Irradiation; Gamma radiation polymerization; Monomers
Download this article as:| Copy the following to cite this article: Sadeghi M, Shafiei F, Mohammadinasab E. Effect of Chemical Parameters on the Properties of Hydrogels Prepared by using Gamma Radiation Polymerization. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Sadeghi M, Shafiei F, Mohammadinasab E. Effect of Chemical Parameters on the Properties of Hydrogels Prepared by using Gamma Radiation Polymerization. Available from: http://www.orientjchem.org/?p=22712 |
Introduction
Hydrogels are materials that exhibit the ability to swell in water and retain significant fractions of water within the structure. Their ability to absorb water is due to the presence of hydrophilic groups such as OH,CONH2,CONH–,COOH and SO3H.[1]Applications of hydrogels have received considerable attention by many researchers in a variety of fields. These fields include enzyme immobilization,biosorbents in preparative chromatography, and the development of various biomedical systems ranging from biosensors to artificial muscles.[3] Two other fields in which hydrogels are prominent include site-specific drug delivery systems [4–12] and superabsorbent polymers.[11,12] The application of radiation techniques to obtain polymeric materials for different purposes began in the last few years. Electron beam irradiation of aqueous solutions containing appropriate monomer mixtures such as acrylamide, acrylic acid, and vinyl acetate were prepared and used as flocculates for waste water treatment and for agriculture and medicine.[13] The hydrogels based on polyelectrolyte structure have been prepared using gamma radiation of aqueous solutions of hydrophilic monomers. Aqueous solutions of acrylamide and N-vinyl-2-pyrrolidone with small amounts of maleic acid and itaconic acids were regarded as systems with potential immobilization, chelating and adsorptive properties for various bioapplications. Thus, Bovine Serum Albumin was investigated by using gels with varying compositions of maleic acid[14] and itaconic acid moieties.[15] The wound dressings manufactured by the simultaneous radiation induced crosslinking and sterilization of hydrophilic polymers were produced by Rosiak et al.[16] This indicates that the composition of hydrogels is very essential in determining its applications and its efficiency. Thus, the present work deals with the radiation-induced polymerization of acrylic monomers in the presence of,N, N’-methylene bisallyamide as crosslinking agent.
Materials And Methods
Material
Hydrolyzed collagen (Parvar Novin-E Tehran Co.) was industrial grade which is available in market and has nearly 25% insoluble phosphate salt. 2-acrylamido-2-methylpropanesulfonic acid (Merck, Darmstadt, Germany), Acrylic acid(Fluka) were of analytical grade and used without further purification.
Determination of soluble fraction
The samples of the hydrogels were placed in a beaker containing bidistilled water and then boiled for 4 hr. The swelled pieces were taken and dried in a vacuum oven at 60°C to remove water. The drying was continued till constant weight, W1. The soluble fraction was calculated according to the following equation:
where W0 is the initial weight of the sample and W1 is the weight of the dried sample after extraction of soluble fraction.
Determination of water uptake
After extraction of the soluble part, a certain weight (W1) of the hydrogel was placed in a beaker containing bidistilled water for 24 hr at room temperature (20°C). The sample was removed from the beaker and blotted with filter paper just to remove the droplets of water on the surface. The water uptake of the sample was calculated using the following equation:
where W1 is the initial weight of the hydrogel and W2 is the final weight of the swelled hydrogel.
Results And Discussion
Grafting mechanism
A general reaction mechanism for graft copolymerization of AMPS onto collagen backbones in the presence of gamma radiation is shown in Scheme 1. In the first step, the gamma radiation, with dose of 35 kGy, produce the radicals from one of the functional groups (i.e. COOH, SH, OH, and NH2) in side chains of the collagen backbones. Then these macroradicals initiate grafting of monomers onto collagen backbones leading to a graft copolymer. Since a crosslinking agent, e.g. MBA, is presented in the system, the copolymer comprises a crosslinked structure that has called superabsorbent hydrogel[17].
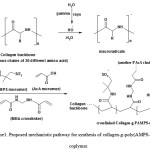 |
Scheme1. Proposed mechanistic pathway for synthesis of collagen-g-poly(AMPS-co-AcA) coplymer. Click here to View scheme |
Effect Of Monomer Ratio On Swelling Capacity
The swelling capacity of the hydrogels prepared with various ratios of monomers is shown in Fig. 1. Since pH of the polymerization mixture was adjusted at 8.0 after the reaction, the superabsorbency of H-protein-g-Poly(AMPS-co-AA) hydrogels is due to both functional groups of ionic sulfonate and carboxylate (from neutralized AMPS and AA, respectively). The presence of the ionic groups in polymer chains results in increasing of swelling because the ionized groups are more strongly solvated rather than ionic groups in the aqueous medium. Higher swelling capacities are obtained from employing higher initial ratios of AMPS/AA. Therefore, the swelling enhancement versus higher AMPS/AA. ratio can be attributed to the formation of high sulfonate and carboxylate groups in the synthesized samples[17-18].
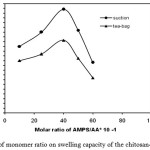 |
Figure :1 Effect of monomer ratio on swelling capacity of the chitosan-based hydrogels. Click here to View figure |
Effect Of Crosslinker Concentration
Figure 2 depicts the effect of N, N’-methylene bisallyamide (MBA) concentration on the swelling capacity of the hydrogels. As shown in the figure, the higher the crosslinker concentration, the lower water and saline absorbencies will be. This well-known relationship between the swelling ratio and concentration of the crosslinking agent is stated as Eq. (3) [22].
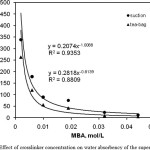 |
Figure :2 Effect of crosslinker concentration on water absorbency of the superabsorbent. Click here to View figure |
where k and n are constant values for an individual hydrogel. Fig. 5 exhibits a power law absorbency-[MBA] behavior, with k = 0.20 and n = 0.93 in the case water and k = 0.28 and n = 0.88 in the case of the saline solution which is obtained from the curve fitted with Eq. 3. Higher crosslinker concentration produces more crosslinked points in the polymeric chains and increases the crosslinking extent of the polymer network, which limits the swelling when it is brought into contact with the swelling medium. However, with N, N’-methylene bisallyamide (MBA) concentrations lower than 0.002 mol/L, no hydrogel with good dimensional stability (swollen gel strength) was prepared. Therefore, maximum swelling capacity in distilled water with suction(338 g/g) and with tea-bag (263 g/g) was achieved at 0.002 mol/L of N, N’-methylene bisallyamide (MBA)[19-20].
Effect Of Reaction Time
Figure 3 demonstrates the swelling variations in lieu of time of the crosslinking reaction. At the early minutes of the reaction, the swelling of hydrogel is around 100-150 g/g. It is gradually increased to about 352g/g after 140 min. Meanwhile, the swelling capacity of the hydrogel prepared after this time is disappreciably decreased in longer reaction time due to enhancement of the crosslinking extent. No remarkable change of swelling capacity was observed in the case of longer time of the reaction[21-22].
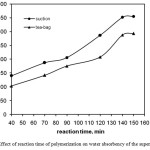 |
Figure :3 Effect of reaction time of polymerization on water absorbency of the superabsorbent. Click here to View figure |
Effect of irradiation condition
Usually, the radiation polymerization is carried out under inert atmosphere such as nitrogen. In this work a comparison has been made between the radiation polymerization in air and under inert gas. A representative mixture of AMPS/AA in the ratio (3:1) was taken and the radiation dose was 15-50 kGy area. The results obtained showed that the swelling capacity of the hydrogel obtained was 445g/g in air and 178g/g in nitrogen atmosphere. These values are practically the same and the difference between them is within experimental error. For this reason all the radiolytic polymerization reactions have been carried out in nitrogen[23].
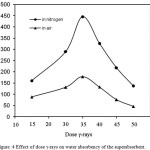 |
Figure :4 Effect of dose γ-rays on water absorbency of the superabsorbent. Click here to View figure |
Conclusions
After making synthesis superabsorbent hydrogel based on H-protein-g-poly(AMPS-co-AA), the reaction conditions were attempted to optimize for obtaining graft copolymers with higher swelling capacity. So, the reaction conditions for achieving the maximum water absorbency(450 g/g) were found to be as follows: γ-rays 35 kGy ,molar ratio AMPS/AA 4.0 , MBA 0.002 mol/L and reaction time 140 min. The swelling behavior of the superabsorbents prepared from NaAMPS and AA indicated that the water absorbency was improved by the addition of a small amount of the AA monomer into the copolymeric gels.
References
- F. L. Buchholz and A. T. Graham, in Modern Superabsorbent Polymer Technology, Wiley, New York, (1997).
- United States Department of Agriculture, US Patent,3 981 100(1961).
- R. Po, J,. Macromol. Sci.-Rev. Macromol. Chem. Phys.34, 607(1994).
- L. P. Krul, E. I. Narciko, Y. I. Matusevich, L. B. Yakimtsova, V. Matusevich, and W. Seeber,. Polym. Bull.45, 159(2000).
- F. A. Dorkoosh, J. Brussee, J. C. Verhoef, G. Borchard, M. Rafeiee-Tehrani, and H. E. Juninger,. Polymer,41, 8213 (2000).
- K. M. Raju, M. P. Raju, and Y. M. Mohan,.J. Appl. Polym. Sci.85, 1795(2000)
- D. W. Lim, K. J. Yoon, and S. W. Ko,. J. Appl. Polym. Sci.78, 2525(2000).
- J. Kost, in Encyclopedia of Controlled Drug Delivery; E. Mathiowitz, Ed., Wiley, New York, Vol. 1, p. 445(1999).
- N. A. Peppas, and A. G. Mikes,. in Hydrogels in Medicine and Pharmacy; CRC Press, Boca Raton, Florida, Vol. 1(1986).
- A. S. Hoffman,. in Polymeric Materials Encyclopedia. J.C. Salamone (Ed.), CRC Press, Boca Raton, Florida, Vol. 5, p. 3282(1996)
- M. Yazdani-Pedram, J. Retuert, and R. Quijada, Macromol., Chem. Phys.201, 923 (2000).
- Y. Sugahara, and O. Takahisa,. J. Appl. Polym. Sci. 82, 1437(2001)
- G. M. Patel, and H. C. Trivedi,. Eur. Polym. J.35, 201 (1999).
- S. Silong, and L. Rahman, J. Appl. Polym. Sci.76, 516(2000 ).
- R. Lapasin and S. Pricl,. in Rheology of Industrial Polysaccharides, Theory and Applications, Blackie, Glasgow, p. 31(1995).
- M. Yalpani,. in Polysaccharides Synthesis, Modifications and Structure/Property Relations, Elsevier, New York, p. 10(1998).
- J. A. Rowley, G. Madlambayan and D. J. Mooney,. Biomaterials, 20, 45(1999) .
- A. Martinesen, I. Storro and G. Skjak-Braek,. Biotech. Bioeng.39, 186(1992) .
- G. R. Mitchell and J. M. V. Blanshard,. Texture Studies, 7, 219(1976).
- L. B. Peppas, and R. S. Harland, in Absorbent Polymer Technology; Elsevier, Amsterdam, (1990)
- P. J. Flory, in Principles of Polymer Chemistry; Ithaca, Cornell University Press, New York, (1953).
- W. F. Lee, and G. H. Lin,. J. Appl. Polym. Sci.79, 1665(2001).
- V. D. Athawale, and V. Lele,. Carbohydr. Polym. 35, 21 (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









