Dissolution Study of Some Antibiotics
Pinki Sharma and L. K. Tiwary*
Regional Institute of Education, NCERT, Bhopal - 462 001, India.
Article Received on :
Article Accepted on :
Article Published : 24 Oct 2016
Dissolution behaviour of pure cefprozil monohydrate (CM) and cefuroxime axetile (CA) and their marketed tablets have been studied in aqueous as well as micellar media. Dissolution of cefprozil monohydrate is significantly assisted in presence of sodium dodecyl sulphate (SDS) and cetyltrimethylammonium bromide (CTAB). But, SDS and CTAB behave differently in accelerating the dissolution tendency of pure CM and its marketed tablet. Effect of SDS in enhancing the dissolution value of CM tablet is found to higher than that of CTAB whereas this dissolution trend is reversed with pure CM. In case of CA dissolution values are found to be appreciably higher in micellar media as compared to the values in aqueous media. However, SDS shows higher impact in enhancing the dissolution values of CA as compared to that of CTAB for both pure CA and its marketed tablet.
KEYWORDS:Cefprozil monohydrate; Cefuroxime axetile; Percentage dissolution; Sodium dodecyl surphale; Cetyltrimethylamonomum bromide
Download this article as:| Copy the following to cite this article: Sharma P, Tiwary L. K. Dissolution Study of Some Antibiotics. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Sharma P, Tiwary L. K. Dissolution Study of Some Antibiotics. Available from: http://www.orientjchem.org/?p=22885 |
Introduction
In the most common situation, a tablet is ingested and passes through the esophagus to the stomach. Because the stomach is an aqueous environment, this is the first place where a tablet may dissolve. The rate of dissolution is a key target for controlling the duration of a drug’s effect and as such several dosage forms that contain the same active ingredient may be available, differing only in the rate of dissolution. If a drug is supplied in a form that is not readily dissolved, the drug may be released more gradually over time with a longer duration of action. Having a longer duration of action may improve compliance since the medication will not have to be taken as often. Additionally, slow-release dosage forms may maintain concentrations within an acceptable therapeutic range over a long period as opposed to quick-release dosage forms which result in sharper peaks and trough in serum concentrations.
Drugs are almost never administered as such to the body, but as formulations containing many ingredients presumed to be inert. Surfactants used as emulsifying agents, solubilizers, stabilizers or as wetting agents in formulations can not be considered to be inert additives as they can lead to significant changes in the biological activity of active ingredients in the formulation1.
Utilization of a drug involves its release from the formulation, its solution in the body fluids, and its passage through barrier membranes into the systemic blood stream before transport into tissues and eventual arrival at the target organ. Release of poorly soluble drugs from tablets or capsules for oral use may be increased by the presence of surface active agents, which may decrease the aggregation of drugs particles and therefore increase the area of particle available for dissolution. Above critical micelle concentration (CMC) the increase in the saturation solubility of the drug by solubilization in the surfactant micelles can result in more rapid rates of drug solution. Since dissolution is the rate-limiting step in the absorption process an increase in the rate of solution will increase the rate of drug entry into the blood and may effect peak blood levels. However, very high concentrations of surfactant in excess of that required to solubilize the drug can decrease drug absorption by decreasing the chemical potential of the drug.
Some surfactants have a direct physiological activity of their own and in the intact animal can thus effect the physiological environment such as by altering gastric residence time such that without physico-chemical intervention, a surfactant effect may be seen. Numerous studies on the influence of surfactants on drug absorption have shown them to be capable of increasing, decreasing, or exerting no effect on the transfer of drugs across biological members2-5.
In general, surfactants play an important role in contemporary pharaceutical chemistry because they are largely utilised in various drug dosage forms to control wetting, stability, bioavailability, among other properties6-7. The lyophobic colloids such as polymers require certain energy to be applied for their formation, are quite unstable from the thermodynamic point of view, and frequently form large aggregates. Association colloids such as micelles, on the other hand, can form spontaneously under certain conditions and are thermodynamically more stable towards both dissociation and aggregation8. Cefprozil monohydrate (I) and cefuroxime axetile (II) are common antibiotic used in the treatment of respiratory and urinary tract infections.
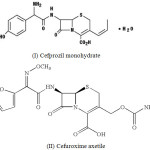 |
Scheme 1 |
We have made a detailed investigation of the aggregation behaviour of CM & CA in aqueous as well as micellar media9-10. The present piece of research work aims to explore the dissolution behaviour of CM & CA in their available dosage form in aqueous and micellar media and also to compare these results with results obtained with their pure active ingredients.
Material and Methods
Absorption maximum of CM and CA are found to be 242 nm and 281 nm respectively in acidic medium. Calibration curve of both CM and CA are plotted by determining absorbance’s of solutions having 5, 10, 15, 20, 25 mg/mL concentration. Slope of the curve was determined using regression equation. For dissolution study a volume of 900 mL was taken in the dissolution study. A fixed volume of solution was withdrawn from dissolution apparatus at regular interval of time and their absorbances were determined. The percentage dissolution (uncorrected) was determined by the relation.
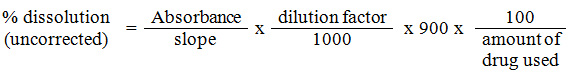
In both dissolution studies of CM and CA dilution factor dilution factor used is 20 and in both cases 250 mg tablets were utilised for measuring their dissolution value. Correction factor is calculated using the relation.
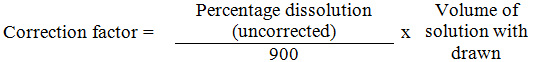
The factor is added in the next uncorrected percentage dissolution to get corrected percentage dissolution. All measurement were carried out in acidic environment and at 37oC to maintain appropriate Gastreointestinal (GI) fluid environment. Both dissolution apparatus and uv-visible spectrophometer used are of LABINDIA MAKE. The pure compounds CM and CA are obtained from LUPIN as gift samples.
Results and Discussion
Since all measurement are carried out in GI environment the absorption maximum (lmax) of CM & CA have also determined in mild acidic condition (0.1 N) and are found to be 242 and 281 nm. The regression equations utilized in the calibration curve of CM are CA furnished their slopes as 0.04 and 0.027 respectively. The complete dissolution study may be subdivided into two parts.
Dissolution study of CM
Percentage dissolution of CM have been calculated by determining absorbance of drug solution from dissolution apparatus at regular interval. After adding correction factor the corrected percentage dissolution have been calculated. The data have been presented in Table 1 and Table 2 for CM 250 mg. tablet and pure CM compound.
Table 1 : Corrected percentage dissolution of CM tablet
(RPM = 50, volume = 900 mL)
|
Time (minutes) |
Absorbance |
Corrected % dissolution |
||||
|
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
|
5 10 15 20 25 30 40 45 |
0.115 0.245 0.335 0.371 0.398 0.432 0.511 0.550 |
0.172 0.312 0.400 0.460 0.467 0.496 0.548 0.559 |
0.174 0.309 0.429 0.512 0.548 0.570 0.575 0.564 |
20.7 44.33 61.02 68.17 73.77 80.69 95.77 103.81 |
30.96 56.50 72.97 84.57 86.75 92.90 102.45 105.53 |
31.32 55.97 78.18 93.98 101.49 106.54 107.73 106.89 |
Table 2 : Corrected percentage dissolution of pure CM compound
(RPM = 50, Dissolution Volume = 900 mL)
|
Time (minutes) |
Absorbance |
Corrected % dissolution |
||||
|
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
|
5 10 15 20 25 30 40 45 |
0.11 0.226 0.318 0.250 0.536 0.548 0.550 0.570 |
0.152 0.248 0.545 0.545 0.587 0.578 0.573 0.570 |
0.205 0.309 0.406 0.438 0.501 0.542 0.562 0.579 |
19.80 40.90 57.91 77.79 98.28 101.52 102.34 107.04 |
27.36 44.94 98.90 99.99 108.64 108.19 107.36 107.97 |
20.7 56.03 74.11 80.68 92.89 101.28 105.96 110.15 |
As expected, dissolution of CM gradually increases with time and is assisted in the presence of SDS and CTAB. This assistance by SDS is observed to more than that of CTAB in the case of CM tablet but trend is reversed for pure CM. In the dosage form drug release kinetics is highly influenced by the presence of surfactants whose concentration remain above their CMC. The micelles suppresses the aggregation of active ingredient of drug thereby gradually increasing their dissolution rate. Whereas pure CM has higher tendency to remain in anionic form. Even in the acidic medium this ionisation is suppressed but appreciable concentration of anionic form is supposed to remain in the solution. The repulsion among the anionic drug species and anionic surfactants eventually favour the drug aggregation and thereby decreasing the dissolution.
Dissolution study of CA
Absorbance values of marketed 250 mg CA tablet and its pure active ingredient have been measured by withdrawing solution from dissolution apparatus at regular interval. From absorbance values percentage dissolutions are calculated and after adding correction due to regular withdrawal in the next dissolution values corrected percentage dissolutions are obtained. The data is presented for CA tablet and pure CA compound in table 3 and Table 4.
Table 3 : Corrected percentage dissolution of CA tablet
(RPM = 50, Dissolution volume = 90 mL)
|
Time (minutes) |
Absorbance |
Corrected % dissolution |
||||
|
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
|
5 10 15 20 25 30 40 45 |
0.110 0.221 0.301 0.350 0.360 0.365 0.372 0.373 |
0.147 0.265 0.32 0.359 0.375 0.378 0.385 0.390 |
0.152 0.273 0.345 0.375 0.374 0.379 0.376 0.386 |
29.33 59.09 80.59 93.77 96.51 97.86 99.74 100.01 |
39.20 70.88 86.21 96.82 101.62 102.97 104.92 106.83 |
40.53 73.02 92.63 101.14 101.43 103.32 102.57 105.79 |
Table 4 : Corrected percentage dissolution of CA
(RPM = 50, Dissolution Volume = 900 mL)
| Time (minutes) | Absorbance | Corrected % dissolution | ||||
|
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
|
5 10 15 20 25 30 40 45 |
0.301 0.325 0.370 0375 0.372 0.375 0.372 0.373 |
0.245 0.297 0.324 0.389 0.395 0.379 0.376 0.386 |
0.283 0.326 0.358 0.390 0.387 0.386 0.384 0.387 |
80.30 87.10 99.10 100.51 99.80 100.62 99.80 100.60 |
65.33 79.56 87.20 105.02 107.19 103.52 102.79 106.02 |
75.47 87.35 96.37 105.43 105.21 105.52 105.02 106.39 |
It is evident from the Table 3 and Table 4 that surfactants are playing a significant role in dissolution process, particularly in the dosage form. SDS seems to be more efficient in enhancing the dissolution process for both CA tablet and pure CA ingredient. If we see that structure of CA it can be realised that the molecule has several sites of protonation. The protonated CM formed in acidic medium are easily attracted in the micellar environment of SDS and their aggregation tendency is reduced thereby dissolution process is significantly favoured. For pure CA dissolution values are initially lowered in presence of surfactants but with passage of time dissolution values in micellar media dominate over the values in aquous media. The reason may be complex interaction between drug molecules and surfactant aggregates initially thereby decreasing the absorbance of CA in the micellar media. But, after proper agitation the chromophone might become free and absorbance values are significantly increased. Crystal lattice energy may also have higher value requiring more time and energy for dissolution process.
Conclusion
Micellar media is found to be significantly assisting the dissolution process of both CM and CA. Both SDS and CTAB are found to be effective in accelerating the dissolution rate of CM as compared to its dissolution in aqueous media. However, SDS is found to more more effective to enhance the dissolution of CM in its dosage form as compared to that of CTAB. But this tendency is just opposite in case of pure CM. In case of CA, again SDS & CTAB both accelerate the dissolution process in its dosage form but they are seen to have some interaction with pure CA in initial stage and dissolution value is lowered. But, with passage of time dissolution values in presence of micelles dominate over the values in aqueous solution.
Acknowledgement
Principal, RIE, Bhopal is thankfully acknowledged for providing laboratory facilities.
Authors are thankful to Lupin, Mandideep, Raisen for providing pure samples of CM & CA as gift.
References
- Florence, A.T., Pure & Appl. Chem., 53, 2057. (1981).
- Eleworthy, P.H., Florence, A.T. & Macfarlane, C.B., Solubiolization by surface active agents, Chapman & Hall, London (1968).
- Billard, G. & Dielafe, L., Comp. Rend. Soc. Biol, 56, 146 (1904).
- Florence, A.T. in Yalkowsky, S (Ed.), Techniques of Solubilization of Drugs, Dekker, New York (1981).
- Pratt, R.S. & Cook, G.M.W., Biochem. J., 179, 299 (1979).
- Torchilin, V.P., J. Control Red., 73, 137 (2001).
- Martin, A., Physical Pharmacy, 4th Ed., Williams & Wrikkins, Baltimore, USA (1993).
- Hunter, R.J., Introduction to Modern Colloid Science, Oxford University Press, Oxford (1993).
- Sharma, P. & Tiwary, L.K., Oriental J. Chem., 26 (4), 1491 (2010).
- Sharma, P. & Tiwary, L.K., Oriental J. Chem., 28 (2), 1043 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









