Determination of Some Properties of Reused Cooking Ground Nut Oil using FTIR Spectroscopy
J. Thamaraiselvi and S. Kalpanadevi
Department of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore - 641 043, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Depending upon temperature and the duration of the deep-frying process, the heating of fats and oils will change the composition of the medium and eventually lead to the degradation of the fat. In the present work we can use Groundnut oil to different temperature conditions and analyses using FT IR spectroscopy.
KEYWORDS:Reused Cooking; Nut; Oil
Download this article as:| Copy the following to cite this article: Thamaraiselvi J, Kalpanadevi S. Determination of Some Properties of Reused Cooking Ground Nut Oil using FTIR Spectroscopy. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Thamaraiselvi J, Kalpanadevi S. Determination of Some Properties of Reused Cooking Ground Nut Oil using FTIR Spectroscopy. Available from: http://www.orientjchem.org/?p=22744 |
Introduction
Peanut oil also known as groundnut oil, is a mild tasting vegetable oil derived from peanuts. The oil is available in refined, unrefined, cold pressed and roasted varieties, the latter with a strong peanut flavour and aroma, analogous to toasted sesame oil. It is often used in Chinese, South Asian and Southeast Asian cuisine, both for general cooking, and in the case of roasted oil, for added flavour. Peanut oil has a high smoke point relative to many other cooking oils. Its major component fatty acids are oleic acids (46.8% as olein), linoleic acid (33.4% as a linolein), and palmitic acid (10.0% as palmitin). The oil also contains some stearic acid, arachidic acid, arachidonic acid, behenic acid, lignoceric acid and other fatty acids. Peanut oil is a common oil for frying foods, due to the high smoke point.
Groundnut oil contain heart friendly MUFA( Mono Unsaturated Fatty Acids) that lower the levels of bad cholesterol in our body without lowering the levels of good chelestrol1.
Deep frying is a cooking process, with water containing foodstuff is immersed into edible oils or fats at temperature between 140 – 190°C. In the first phase, within a few seconds, a thin crust forms, whose structure crucially affects the deep-frying process and the quality of the food with regads to fat absorption and crispness2.
With the rise of temperature in the boundary layer to more than 120°C, the formation of acrylamide begins, in particular in the presence of reducing sugars and asparagines like in grain- or potato products3.
Depending upon temperature and the duration of the deep-frying process, the heating of fats and oils will change the composition of the medium and eventually lead to the degradation of the fat. In the present work we can use Groundnut oil to different temperature conditions and analyses using FT IR spectroscopy.
Materials and Methods
Samples and Standards
Groundnut oil acquired from a local super market. This oil sample without heating and it was analyzed using FTIR spectroscopy (oil 1)
Heating of groundnut oil
10ml of groundnut oil was heated to 185°C in a stainless steel deep fryer for 1 hour without stirring or agitation and it is kept at a constant temperature and the oil was allowed to cool at room temperature until the next heating session start. 10ml of oil withdrawn from the deep fryer and it was analyzed using FTIR spectroscopy. The same procedure was repeated for 4 times (i.e) oil-2, oil-3, oil-4, oil-5.
Continuous heating of groundnut oil
The remaining portion of groundnut oil was heated to 185°C in a stainless steel deep fryer for 6 hour without stirring or agitation. Then the 10 ml of oil sample were immediately analyzed by using FTIR spectrophotometer (i.e) oil-6.
Table 1: Different types of groundnut oil prepared
| S.No | Oils | Types |
| 1 | Groundnut oil 1 | Without heating of groundnut oil- oil 1 |
| 2 | Groundnut oil 2 | One hour heating of same groundnut oil at frying temperature -oil 2 |
| 3 | Groundnut oil 3 | Again one hour heating of same groundnut oil at frying temperature -oil 3 |
| 4 | Groundnut oil 4 | Again one hour heating of same groundnut oil at frying temperature -oil 4 |
| 5 | Groundnut oil 5 | Again one hour heating of same groundnut oil at frying temperature -oil 5 |
| 6 | Groundnut oil 6 | Again 6 hour heating of same groundnut oil at frying temperature -oil 6 |
Results and discussion
By comparing the standard spectrum of the groundnut oil (i.e) spectrum of oil 1 to another five spectrum (i.e) oil-2, oil-3, oil-4, oil-5, & oil-6. We can conclude that what are the changes present in groundnut oil during frying process and also continuous heating process.
The groundnut oil headspace on different types of frying process, it can be observed that one time used groundnut oil headspace contains some components in very low proportions that are also present in similar concentrations on the following process upto 6 times used groundnut oil. This could be due to the degradation of the small amount of the hydroperoxides contained in the oil at the beginning of the experiment.
Table: 2
| Some Common functional group frequencies present in all oils | |
| 4000-4500 cm-1721.40 cm-1723.33 cm-11099.46 cm-11163.11 cm-11263.41 cm-11238.34 cm-11747.57 cm-12332.02 cm-1 | 2679.21 cm-12681.14 cm-12924.18 cm-13009.05 cm-1 |
It is clear from the table 2 that, in all the 6 spectrum, the region between 4000-4500 cm-1 is common harmonic peaks of normal frequency samples containing moisture can also be measure in this region. The region between 700-1000 cm-1, the functional group obtained may be aliphatic chloro compound, C-F stretch, C-O stretching, Inplane bending bands, skeletal C-C vibration, C-O stretching vibration, Inplane bending bands. Then the functional groups obtained in the region between 1300-2000 cm-1 may be Nitrate ion. C-H deformation, carbonate ion, C-H deformation, -CH3, >CH2 C=C stretch, methyl C-H asymmetric / symmetric bend, C=C stretch alkyl carbonate, anhydrides shows 2 strong bonds, C-H stretching mode of olefins and esters. Then the functional groups obtained in the region 2679.21 cm-1 may be C-H stretch, O-H chelate form.Then the functional groups obtained in the region 2854.74 cm-1may be methylene C-H assy/ sym stretch, O-H chelate form. In 2924.18 cm-1 the functional groups may be C-H stretch, O-H chelate form. The functional groups obtained in the region 3009.05 cm-1 may be C-H stretch.
The above mentioned functional groups were common in all 6 oils even at different frying process. From the above discussion we can concluded that during different frying process, some of the components present in groundnut oil do not change.
By comparing the spectrum of oil 1 & oil 2 some of the peaks (or) functional groups may be destroyed and some of the functional groups may be produced, and some of the peaks do not change. The extra functional groups in oil 2 may be 3387.11 cm-1, 3338.89 cm-1, 2542.26 cm-1, 2360.95 cm-1 2278.01 cm-1, 1022.31 cm-1 846.78 cm-1, 669.32 cm-1.
The region between 3300-3400 cm-1 contained functional groups may be hydroxyl group, H-bonded O-H stretch, NH2, > NH. The peak value 1022.31 cm-1 contain functional group may be aliphatic fluoro compounds, C-F stretch, skeletal-C vibrations, C-O stretching vibrations. The peak value 846.78cm-1 contains functional groups may be skeletal C-C vibrations, meta substituted compounds, C-H bending vibrations.The functional groups obtained in 669.32 cm-1 may be alkyl C-H bond.
The above mentioned functional groups may e produced in oil 1. This shows us the extra compounds may be produced during frying process.
Some of the functional groups may be destroyed in oil-2 during frying that are 4579.16 cm-1 , 4212.67 cm-1 , 4170.24 cm-1 , 3468.13 cm-1 , 3419.08 cm-1 , 3198.08 cm-1 , 2679.21 cm-1, 1464.02 cm-1 , 1456.30 cm-1 , 1361.79 cm-1 & 1033.88 cm-1 . These functional groups are original components of the groundnut oil, this components were destroyed in oil 2.
We have already discussed about the functional groups, common in all 6 oil even at different frying process.
When we compare the spectrum of oil 1, oil2 & oil 3 again some of the functional groups may be destroyed, some of the functional groups may be produced and some of the functional groups may be common.
The extra functional groups present in oil 3 may be 4401.70 cm-1, 4201.70 cm-1, 3265.59 cm-1, 2953.12 cm-1, 2193.14 cm-1, these are the functional groups may be contamination of the groundnut oil.
The functional groups in the region 3421.83 cm-1 may beydroxyl group, H-bonded O-H stretch, normal polymeric O-H stretch, -NH2,>NH groups, H-bonded OH. The functional groups in the region 2953.12 cm-1 may be methyl C-H assy/sym bend, C-H deformation, CH3>CH2,C=c stretch. The functional groups in the region 2193.14 cm-1 may be C=C, methyl alkyne, cyanide, cyanates & isocyanates.
By comparing the spectrum of oil 1, oil 2, oil 3, oil 4 the extra functional groups present in oil 4 were 4592.66 cm-1, 4019.79 cm-1, 3300.31 cm-1, 3180.72 cm-1, 1035.81 cm-1. The peak value above 4000 cm-1 containing moisture, and the peaks obtained in 3300.31 cm-1 may be hydroxyl group, H-bonded OH stretch, normal polymeric O-H stretch, NH2>NH,H-bonded OH,N-H stretch. The functional groups obtained in 1035.81 cm-1 may be aliphatic fluoro compounds, C-F stretch, skeletal C-C vibrations, C-O stretching vibration.
When we compare the spectrum of oil 1, oil 2, oil 3, oil 4, oil 5 the extra functional groups present in oil 5 were 3198.08 cm-1. The functional group obtained 3198.08 cm-1 may be ammonium ion, C=H stretch, Ar-H stretch.
Finally when we compare all 6 oils, the oil 6 is the continuous heating of same groundnut oil upto 6 hours. The extra functional groups present in oil 6 may be 4579.16 cm-1,4046.79 cm-1, 1377.22cm-1 580.59 cm-1. The extra functional groups obtained in 1377 cm-1 may be carbonate ion, C-H deformation, CH3,>CH2,C=C stretch. The extra functional groups present in oil 6 may be contaminates (or) toxins of that groundnut oil.
The region between 3600-3100 cm-1 there is non oxidized oils a band near 3470 associated with overtone of the glyceride ester carbonyl absorption. The band with a maximum frequency near 3440cm-1 due to the presence of hydroperoxides.
The changes are also observed in the band at ~ 3009 cm-1 owing to the stretching vibration of cis-CH olefinic group. After heating to 185°C a clear & pronounced diminution in the frequency value of this band is produced as a consequence of the diminution of cis double bonds. The band at ~ 1654 cm-1 associated with the stretching vibration of the carbon-carbon double bonds of cis olefins. The band at ~1746 cm-1 due to the ester carbonyl functional group of the triglycerides. It should be noted that some of these aldehydes are responsible for the oxidized odours. The frequency of the band originally at ~968 cm-1 which is associated with bending vibrations of CH groups of isolated trans olefins 4-hydroxyl trans -2- nonenal.
 |
Figure 1: FTIR spectrum of the groundnut oil 1 Click here to View figure |
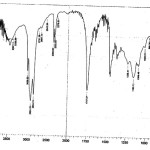 |
Figure 2: FTIR spectrum of the groundnut oil 2 Click here to View figure |
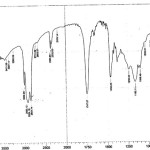 |
Figure 3: FTIR spectrum of the groundnut oil 3 Click here to View figure |
 |
Figure 4: FTIR spectrum of the groundnut oil 4 Click here to View figure |
 |
Figure 5: FTIR spectrum of the groundnut oil 5 Click here to View figure |
 |
Figure 6: FTIR spectrum of the groundnut oil 6 Click here to View figure |
Summary and Conclusion
The present work dealt with the analysis of used groundnut oil by using FTIR spectroscopy. This study shows that the reused cooking oils can also affect our health. By comparing the spectrum of all six oils the region between 3600-3100 cm-1 there is a non oxidized oils a band near 3470 cm-1 associated with overtone of the glyceride ester carbonyl absorption. The band with a maximum frequency near 3440 cm-1 due to the presence of hydroperoxides. The frequency of the band originally at 968 cm-1 which is a associated with bending vibrations of C-H groups of isolated trans olefins 4-hydroxy trans-2-nonenal.
Although reusing cooking oil is a somewhat common practice, it can pose some serious health hazards. The most common danger when recycling cooking oil is that it becomes rancid or spoiled. In addition to having strange flavors and odors, rancid oil may contain possibly carcinogenic free radicals.
References
- Lamba, O.P., S.Lal,M.C.Yappert, M.F.Lou And D.Borchman,. “ Spectroscopic Detection Of Lipid Peroxidation Products And Structural Changes In A Shingomyelin Model System BBA, 1081 : Pg No:181-187
- Yoon J., Han B.S., Kang Y.C., Kim K.W,Yung M.Y., Kwon Y A., Food Chemistry 71 (2000) Pg. No :275.
- Barr, David ; Reynhout,Greg ; Guzinski Measuring Oxidation Of Cooking Oil Using EPR Spin Trapping Short Article From Brucker Biospin.
- Quiles J.,Ramirez – Tortosa M.C., Gomez J.A., Huertas J.A ., Mataix J ., Food Chemistry 76 (2002) Pg .No :461.
- U.S. Food And Drug Administration “Irradiation In The Production, Processing And Handling Of Food”.Final Rule. Federal Register 62 (1997) Pg. No: 64107 – 64121.
- P.S. Kalsi, “Spectroscopy Of Organic Chemistry”, New Age International Publications, VI- Edition
- WWW.Google.Com.

This work is licensed under a Creative Commons Attribution 4.0 International License.









