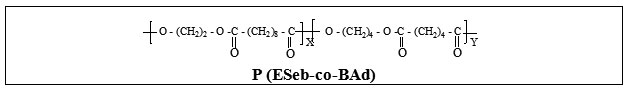
Design, Synthesis and Characterisation of Novel Biodegradable Aliphatic Copolyesters- Poly(ethylene sebacate-co-butylene succinate) and Poly(ethylene sebacate-co-butylene adipate)
L. Sowbagya lakshmi Prabha, R. Nanthini and K. Prabhu
1Department of Chemistry, DG Vaishnav College, Arumbakkam, Chennai - 600 106, India.
Article Received on :
Article Accepted on :
Article Published : 20 Oct 2016
Synthesis of novel aliphatic biodegradable copolyesters namely Poly (ethylene sebacate-co-butylene succinate) and Poly (ethylene sebacate-co-butylene adipate) were carried out using Poly (butylene Succinate), Poly (Butylene Adipate) and Poly (Ethylene Sebacate) in presence of Poly Phosphoric acid. Synthesis of Poly (Butylene Succinate), Poly (Butylene Adipate) and Poly (Ethylene Sebacate) were carried out using respective diols and diacids in presence of titanium tetrabutoxide as catalyst. Newly synthesized copolyesters were characterized by solubility studies, viscosity measurements, thermal analysis, IR Spectroscopy and Nuclear Magnetic Resonance Spectroscopy (1H-NMR and 13C-NMR). The characteristic peaks present in the homopolyesters were recorded in the copolymers as well. DSC analysis of the copolyesters showed multiple melting peaks, while increase of methylene units in the di-acid decreased the Tg.
KEYWORDS:Aliphatic polyester; polycondensation; Poly(ethylene sebacate-co-butylene succinate); Poly(ethylene sebacate-co-butylene adipate); differential scanning calorimetry; spectral analysis
Download this article as:| Copy the following to cite this article: Prabha L. S. L, Nanthini R, Prabhu K. Design, Synthesis and Characterisation of Novel Biodegradable Aliphatic Copolyesters- Poly(ethylene sebacate-co-butylene succinate) and Poly(ethylene sebacate-co-butylene adipate). Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Prabha L. S. L, Nanthini R, Prabhu K. Design, Synthesis and Characterisation of Novel Biodegradable Aliphatic Copolyesters- Poly(ethylene sebacate-co-butylene succinate) and Poly(ethylene sebacate-co-butylene adipate). Available from: http://www.orientjchem.org/?p=22682 |
Introduction
With the use of plastics in large quantities world over for use of convenience, the environmental pollution has also reached gigantic proportion. This has led to a growing interest in search of biodegradable and biocompatible polyesters. In recent years, biodegradable polymers have attracted considerable attention as green materials and biomaterials in pharmaceutical, medical and biomedical engineering applications including drug delivery systems, artificial implants and functional materials in tissue engineering. Among synthetic polymers aliphatic polyesters have attracted considerable attention as they combine the features of biodegradability, biocompatibility and physical or chemical properties comparable with many traditional and non-biodegradable polymers such as low density polyethylene (LDPE) and polypropylene (PP). Biodegradable final products made from these polymers find a variety of end uses especially as films for packaging and in agricultural applications.[1-3] which would partially solve the problem of plastic waste accumulation.
Aliphatic polyesters are one of the most promising biodegradable materials because they are readily susceptible to biological attack. Many new uses such as absorbable bone plates, some surgical fixation devices, bioadsorbable surgical sutures and carriers for the controlled release of drugs have been found. Limiting factors for a broader use of synthetic aliphatic polyesters derived from diols and diacids are their poor mechanical properties and low melting temperature. At the same time, the high hydrolytic instability of these polymers resulted in a multitude of applications for this polymer class in the biomedical field starting with absorbable sutures in the 1960s [4]. This new avenue revitalized interest in this class of polymers, and novel synthetic methods, as well as catalytic systems, were developed to obtain high molecular weight polymers with narrow molecular weight distributions [5-9] . Polyesters are now synthesized by the polycondensation of diacids and diols, self-polycondensation of hydroxyacids, or by the ring-opening polymerization of cyclic diesters, lactones, glycolides, and lactides.
Two classes of polyesters were prepared, based on hydroxycarboxylic acids and on alkanediols and aliphatic dicarboxylic acids. Among them aliphatic polyesters like poly(l-lactic acid), poly(ε-caprolactone), poly(3-hydroxybutyrate), poly(3-hydroxy valerate), etc., are prepared by ring-opening polymerization of lactones or other cyclic esters, whereas the second class of polyesters such as poly(butylene succinate),poly (butylene adipate ) etc., are usually prepared by polycondensation of diols and diacids, consisting a very important class due to their favourable features of biodegradability and biocompatibility [10-14]. Till recently dominated polyesters are derived from ethylene-glycol and 1,4-butanediol, due to difficulties in the preparation of 1,3-propanediol (1,3-PD) in sufficient quantities and purity [15,16]. While many studies are available on poly ( butylene succinate), poly ( butylene adipate) , the use of esters of sebacic acid have not been reported largely[17-22] .
Sebacic acid is an intermediate product of ω- oxidation of long-chain aliphatic acids. Compared with short-chain aliphatic acid, sebacic acid is more suitable for the preparation of polyesters, as short-chain aliphatic acids always conduce to intramolecular condensation. Poly(Ethylene Sebacate) is chosen as one homopolymer to copolymerize with other homopolymers, Poly(Propylene Succinate) and Poly(Propylene Adipate).
Consequently, it is planned to initially synthesise and characterize two homopolymers Poly (Butylene Succinate)(PBSu) and Poly(Butylene Adipate) (PBAd) and then to carryout copolymerization of these homopolymers with Poly(Ethylene Sebacate) (PESeb).
Experimental
Materials
Sebacic acid (99%), Succinic acid (99%), Adipic acid (99%),were purchased from Aldrich Chemical Co. and used as such. Ethylene glycol (purity ≥ 99.7%) and butanediol (purity ≥ 99.7%) were supplied by Merck Co. and used as such.
Synthesis of Polyesters
Synthesis of poly(ethylene sebacate-co-butylene succinate)
Synthesis of aliphatic copolyesters was carried out by two stage melt polycondensation method in a three necked round bottom flask. At the first stage, the oligomers were prepared using sebacic acid or succinic acid and ethylene glycol or butanediol in a molar ratio 1/1.2 and the catalyst titanium tetrabutoxide (3x 10-4 mol TBT /mol Sebacic acid) was charged into the reaction flask of the polycondensation apparatus. The reaction mixture was heated at 190°C under nitrogen atmosphere and stirred at a constant speed, 500 rpm. The first step, esterification is considered to be complete after the collection of theoretical amount of water, which was removed from the reaction mixture.
In the second polycondensation step, oligo(ethylene sebacate) and oligo(butylene succinate) were used in 1:1 weight ratio to synthesize P(ESeb-co-BSu) copolymer. In this stage, polyphosphoric acid, PPA, which is believed to prevent side reactions such as etherification and thermal decomposition, was added (5×10-4mol PPA/mol SebA). A vacuum, 5.0 Pa was applied slowly over a period of about 30 min, to avoid excessive foaming and to minimise oligomer sublimation, a potential problem during the melt polycondensation. The temperature was slowly increased to 230°C, while stirring speed was also increased to 720 rpm. The polycondensation was continued for about 60 min. At the end of polycondensation reaction, the polyester obtained was easily removed, milled and washed with methanol.
Synthetic route to P(ESeb-co-BSu)copolymers preparation, via the two step polycondensation.
First step: Esterification
Using the same procedure poly(ethylene sebacate-co-butylene adipate) was also synthesized.
Polymer characterization
Solubility studies
Solubility of polymers were determined in various organic solvents.10mg of the polyester was taken in a small stoppered test tube and 1ml of the solvent was added. The solubility was noted in different solvents.
Viscosity measurements
Inherent viscosity of the copolyesters were measured in chloroform at 30°C ± 1°C using Ubbelhode viscometer. For this flow times were determined for the pure solvent and 1% polymer solution by weight at room temperature.
Spectral studies
Infrared Spectroscopy
The IR spectra of polymers were recorded using Bruker IFS 66 V – IR spectrophotometer with KBr pellets in the range of 4000-400cm-1 at 25°C,
Nuclear Magnetic Resonance Spectroscopy
1H and 13C NMR spectra of copolyesters were recorded using JOEL-GSX-400 spectrometer. CDCl3 was used as solvent and TMS was used as internal standard.
Thermal analysis
DSC thermograms were recorded on a PERKIN ELMER PYRIS-1 differential scanning calorimeter. About 2-4mg of the polymer sample was heated in an aluminium pan with pierced lid under nitrogen atmosphere at a scanning rate of 10°C/ min between a temperature range of -100°C and 500°C.
Results and discussion
Polyester Synthesis
PESeb, PBSu and PBAd homopolyesters and P(ESeb-co-BSu) , P(ESeb-co-BAd) copolyesters were synthesized by two-step polycondensation reaction.
The polymerisation procedure for the studied polymers involved two different steps according to the well known process used for polyester synthesis. [23,24] .
In the first stage (esterification), selected diacids reacted with diols and water was eliminated as by-product. At the reaction temperature (190°C) water can be easily removed from the reactor and oligomers were formed. In the second stage (polycondensation) the prepared oligomers condensed at a higher temperature (230°C) with the application of high vacuum. The reactions that took place during these stages and the procedures that were used for the synthesis of the studied polyesters are presented in Scheme I & II.
All the synthesized polyesters were purified by dissolving them in chloroform and precipitated in ice-cold methanol to remove low-molecular part as well as traces of the catalyst.
Solubility
The copolyesters are freely soluble in chloroform and carbon tetrachloride, soluble in dichloromethane and tetrahydro furan, sparingly soluble in N, N’-DMS and acetone. The prepared copolyesters are insoluble in water, hexane, diethyl ether and methanol.
Viscosity of the polyesters
The inherent viscosity of the random copolyesters were calculated from the relative viscosity which were obtained from the flow time of the pure solvent and polyester solution using Ubbelohde viscometer in chloroform at 30°Cand at the concentration of 0.10g/dL. The ηinh of P(ESeb-co-BSu) and P(ESeb-co-BAd) obtained in the present work is 0. 22 dL/g and 0. 25 dL/g respectively. Among polymers of comparable molecular weight, rigid polymers possess a higher viscosity value than flexible one .The viscosity data obtained in the present work is analogous to the above mentioned fact.
Spectral studies
The IR spectra have been recorded for all the synthesized copolyesters and are presented in Fig 1-2..
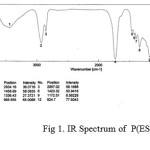 |
figure :1 IR Spectrum of P(ESeb-co-BSu) Click here to View figure |
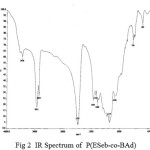 |
Figure :2 IR Spectrum of P(ESeb-co-BAd) Click here to View figure |
The prepared polyesters showed characteristic absorption band for ester carbonyl stretching, C-O-C stretching and methylene groups as shown in the table.1
IR Spectral data of Copolyesters P (ESeb-co-BSu) & P (ESeb-co-BAd) Table 1
| Absorption frequency, cm-1 | Assignment | |
| P (ESeb-co-BSu) | P (ESeb-co-BAd) | |
| 1728 | 1737 | C=O stretching of ester group |
| 1173,1043 | 1171,1071 | C-O stretching of ester group |
| 2934 | 2939 | aliphatic C-H stretching |
| 1459 | 1464 | aliphatic C-C stretching |
1H NMR Spectra
The spectra of the polyesters are presented in fig 3-7.
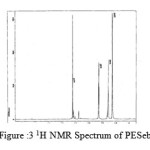 |
figure :3 1H NMR Spectrum of PESeb Click here to View figure |
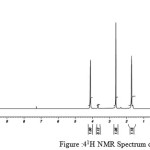 |
figure :41H NMR Spectrum of PBSu Click here to View figure |
 |
figure :51H NMR Spectrum of PBAd Click here to View figure |
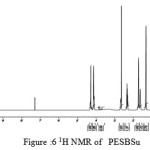 |
figure :6 1H NMR of PESBSu Click here to View figure |
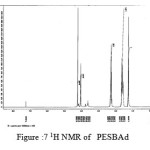 |
figure :7 1H NMR of PESBAd Click here to View figure |
1H NMR spectral data of copolyesters, P (ESeb-co-BSu) and P (ESeb-co-BAd)
The 1H NMR spectra of PESeb showed characteristic peaks at 4.04–4.21 ppm, 2.25 – 2.3 ppm of -O- CH2– of butanediol and -CO-CH2– of sebacic acid respectively. The peaks at 1.64 ppm and 1.24-1.57 ppm are attributed to β and γ methylene protons of sebacic acid. The methylene protons of the succinic acid moiety appeared at 2.63 ppm. β methylene protons of butanediol showed peak at 1.71 ppm.The terminal and central methylene protons of the adipic ester residue appeared at 2.3ppm and 1.62–1.67ppm, respectively. All these characteristic peaks are found in the spectra of copolyesters P(ESeb-co-BSu) and P(ESeb-co-BAd) given in figure (6-7). Copolyester prepared by molten state polycondensation has generally been considered to have a random distribution of the structural units because of the almost equal reactivities of the monomers and the random transesterification reaction during the polycondensation process [25] .
Random chain scission due to ester bond cleavage and formation of new ester bonds occur during polycondensation reaction at elevated temperatures. This leads to formation of random copolymers [26] .
The 13C NMR spectra of copolyesters were recorded in CDCl3 and the relationship between the structure of the monomeric units and the chemical shifts of the carbonyl, –O-CH2-, -CH2 – signals were established. The 13C NMR spectra of PESeb showed signals at 173.66-174.23, 61.12-77.45 and 24.85-34.17 ppm. PBSu spectra showed peaks at 172.32, 64.19-64.57 and 25.05-29.12 ppm. All the characteristic peaks of PESeb and PBSu were also recorded in the copolymer P(BSeb-co-BSu) (fig 11).The 13C NMR spectra of PBAd at 173.41pppm for ester carbon, 63.93 and33.92 corresponds to methylene carbon attached to diol and diacid moiety. 24.44 and 25.36 ppm are attributed to β methylene carbon of diacid and diol. All the characteristic peaks of PESeb and PBAd were also found in the copolyester P(ESeb-co-BAd)(fig 12). Based on these spectral data, it may be concluded that the following structural units are randomly distributed in these copolyesters.
13C NMR Spectra of Oligomers
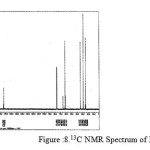 |
figure :8 13C NMR Spectrum of PESeb Click here to View figure |
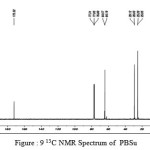 |
figure :9 13C NMR Spectrum of PBSu Click here to View figure |
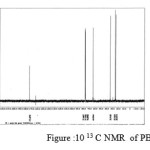 |
figure :10 13 C NMR of PBAd Click here to View figure |
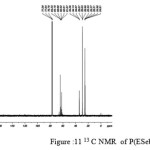 |
figure :11 13 C NMR of P(ESeb- co- BSu) Click here to View figure |
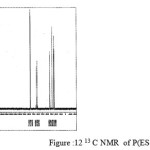 |
figure :12 13C NMR of P(ESeb- co- BAd) Click here to View figure |
Thermal Analysis
The polyesters were further characterised by thermal analysis and the DSC thermograms. DSC thermograms of the synthesized aliphatic polyesters with multiple melting endotherms are shown in Fig. 13-14.These multiple endothermic peaks are attributed to crystallite reorganization during heating [27] and [28] . Crystallites of poor quality and with low melting temperature recrystallize to crystallites with higher melting temperature when low heating rates are employed.
The large area under the melting as well as the small displacement in base line in the Tg region indicate the presence of sufficient degree of crystallinity, which can also be evidenced by the appearance of 3 intense peaks in WAXD study of P (E Seb –co- BSu) as seen in Fig 15. [29].
The melting temperature Tm and the glass transition temperature Tg were found to be 59.5 and 53.5 °C and -47.0and -63.8 °C for the copolyesters P(ESeb-co-BSu) and P(ESeb-co-BAd) respectively. It was found that the melting temperatures and the glass transition temperature decreased with increasing number of methylene units.
| S. No | Polyester | Tm o C | Tg o C |
| 1 | PESBSu | 55.7,71.7,78.5 | -27.1 |
| 2 | PESBAd | 44.2,54.8 | -51.5 |
Thermal Analysis
The polyesters were further characterised by thermal analysis and the DSC thermograms. DSC thermograms of the synthesized aliphatic polyesters with multiple melting endotherms are shown in Fig. 13-14.These multiple endothermic peaks are attributed to crystallite reorganization during heating [27] and [28] . Crystallites of poor quality and with low melting temperature recrystallize to crystallites with higher melting temperature when low heating rates are employed.
The large area under the melting as well as the small displacement in base line in the Tg region indicate the presence of sufficient degree of crystallinity, which can also be evidenced by the appearance of 3 intense peaks in WAXD study of P (E Seb –co- BSu) as seen in Fig 15. [29]
The melting temperature Tm and the glass transition temperature Tg were found to be 59.5 and 53.5 °C and -47.0and -63.8 °C for the copolyesters P(ESeb-co-BSu) and P(ESeb-co-BAd) respectively. It was found that the melting temperatures and the glass transition temperature decreased with increasing number of methylene units
 |
figure :13 DSC Thermogram of P (E Seb –co- BSu) Click here to View figure |
 |
figure :14 DSC Thermogram of P (E Seb –co- BAd) Click here to View figure |
 |
figure :15 WAXD of P (E Seb –co- BSu) Click here to View figure |
Conclusion
In this investigation homopolyesters Poly(ethylene sebacate) PESeb, Poly(butylene succinate) PBSu and Poly(butylene adipate) PBAd and copolyesters Poly(ethylene sebacate-co-butylene succinate) P(ESeb-co-BSu) and Poly(ethylene sebacate-co-butylene adipate) P(ESeb-co-BAd) were synthesised from sebacic acid, succinic acid, adipic acid, ethanediol and 1,4 butanediol by polycondensation in presence of titanium e as catalyst. The chemical composition of di-acid and diol unit had a 1:1.2 mol ratio. Copolyesters Poly(ethylene sebacate-co-butylene succinate) P(ESeb-co-BSu) and Poly(ethylene sebacate-co-butylene adipate) P(ESeb-co-BAd) were synthesized by a two step reaction of esterification and polycondensation. These copolyesters were synthesised with a view of increasing their solubility and improving their mechanical properties. The two copolyesters were found to be freely soluble in chloroform and carbon tetrachloride. The inherent viscosities of these polyesters showed that the polyester containing succinate units had lower viscosity than the polyester containing adipate unit. The probable structure of the repeating units present in these polyesters were assigned on the basis of NMR spectral data. From the DSC thermograms it was shown that the introduction of methylene units in the di-acid decreased the Tg.
References
- Guillet J.In:Scott G, Gilead D, editos. Degradable polymers : principles and applications. London: Chapman &Hall; (1995).
- Albertsson A-C, Ljungquist O.J Macromol Sci Chem; A23:411, (1986).
- Ferre T, Fraco L. Rodriguez-Galan A. Puiggali J. Polymer; 44:6139, (2003).
- Kulkarni RK, Moore EG, Hegyeli AF, Leonard F J, Biomed Matter Res 5:169 (1971)
- Kricheldorf HR, Kreiser-Sauders J, Macromol Chem 191:1057 (1990)
- Jedlinski Z, Walach W, Kurcok P, Adamus G , Macromol Chem 192:2051, (1991)
- Kricheldorf HR , Chemosphere 43:49, (2001)
- Bhaw-Luximon A, Jhurry D, Spassky N, Pensec S, Belleney J,Polymer 42:9651, (2001)
- Kricheldorf HR, Langanke D , Polymer 43:1973, (2002)
- M. Avella, E. Martuscelli, M. Raimo, J. Mater. Sci. 35 ,523, (2000).
- M. Schmid, A. Ritter, A. Gubelnik, M. Zinn, Biomacromolecules, 8,579, (2007).
- M. Zinn, H.U. Weilenmann, R. Hany, M. Schmid, T. Egli, Acta Biotechnol. 2–3, 309, (2003).
- P. Rizzarelli, C. Puglisi, G. Montaudo, Polym. Degrad. Stab. 85, 855, (2004).
- Y. Kumagai, Y. Doi, Polym. Degrad. Stab. 36 , 241, (1992).
- G. Karayannidis, C. Roupakias, D. Bikiaris, D. Achilias, Polymer 44 , 931, (2003).
- B. Wang, C.Y. Li, J. Hanzlicek, S.Z.D. Cheng, P.H. Geil, J. Grebowicz, R.M. Ho Polymer 42, 7171, (2001)
- Jourdan, N.; Deguire, S.; Brisse, F. Macromolecules, 28, 8086–8091, (1995).
- Papageorgiou, G. Z.; Bikiaris, D. N.; Achilias, D. S. Macromol Chem Phys, 210, 90–107, (2009)
- Tang, J.; Zhang, Z.; Song, Z.; Chen, L.; Hou, X.; Yao, K. Eur, Polym J, 42, 3360–3366, (2006).
- Kolwzan, B.; Gryglewicz, S. J Synth Lubric, 20, 99–1,( 2003
- Berti, C.; Celli, A.; Marchese, P.; Marianucci, E.; Barbiroli, G.; Di Credico, F. e-Polymers, 057, ( 2007)
- Medellin-Rodriguez, F. J.; Phillips, P. J.; Lin, J. S. Macromolecules, 28, 7744–7755, 1995
- Karayannidis G. Roupakias C, Bikiaris D, Achillias D. Polymer; 44:931,( 2003).
- Solomon O F ,Cuita Z. J Appl Polym Sci , 6:683, (1962).
- B.D.Ahn, S.H.Kim, Y.H. Kim, J.S. Yang Journal of Applied Polymer Science; vol.82, 2808-2826, (2001)
- George Z. Papageorgiou, Dimitrios N. Bikiaris Macromol. Chem. Phys., 210, 1408-1421, ( 2009)
- R.C. Roberts. J. Polym. Sci., B 8, p. 381, (1970).
- G.E. Sweet and J.P. Bell. J. Polym. Sci.: Part A-2, , p. 1273, (1972).
- G.Z. Papageorgiou, G.P. Karayannidis / Polymer 40, 5325–5332, (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.