Characterization of Transition Metal Complexes Derived from Biologically Active Furoic Acid Hydrazone
Sunil Kumar, Sachin Kumar Singh, Vipul Vardhan and T. R. Sharma
Department of Chemistry, K.G.K. (P.G.) College, Moradabad - 244 001, India.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Biologically active schiff base ligand, N’-2-[(Z)-1-(4-hydroxy-6-methyl-2-oxo-2H-pyranyl)ethylidene]-furancarbohydrazide and its M(III) complexes have been prepared and characterized by elemental analyses, molar conductance, magnetic susceptibility measurement, UV-Vis, IR and NMR spectra. Based on these studies an octahedral geometry has been proposed for these complexes. The ligand and its metal complexes were screened for their antimicrobial activities. The ligand showed higher activity than its corresponding M (III) complexes.
KEYWORDS:Furoic acid hydrazide; antimicrobial activity
Download this article as:| Copy the following to cite this article: Kumar S, Singh S. K, Vardhan V, Sharma T. R. Characterization of Transition Metal Complexes Derived from Biologically Active Furoic Acid Hydrazone. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Kumar S, Singh S. K, Vardhan V, Sharma T. R. Characterization of Transition Metal Complexes Derived from Biologically Active Furoic Acid Hydrazone. Available from: http://www.orientjchem.org/?p=22796 |
Introduction
Furoic acid hydrazones and their metal complexes show varied applications as antifungal (1, 2), antibacterial (3,4) antioxidative (5,6) and cytotoxic (7, 10). These have also proved to be potential chemotherapeutic agents (11,12). In addition to these, furoic acid hydrazones are potential chelating agents and exhibit excellent physiological and biological activities (13, 14). In view of these facts, the present research work has been undertaken.
Experimental
Metal salts, regents and all chemicals used during this research work were of AR grade or equivalent grade and were procured from reputed firms. The solvents used were purified before use. Melting points/decomposition temperatures were determined by open capillary method and are uncorrected. Electrical conductivities were determined at room temperature with freshly prepared solutions of 10–3 M dilution using systronics model 304 conductivity meter. Electronic spectra were recorded using Beckmann DU-spectrometer. The magnetic susceptibility was measured by Gouy’s method using CuSO4.5H2O as calibrant. Carbon, hydrogen and nitrogen were determined by microanalysis at CDRI, Lucknow. The ir spectra were also recorded at CDRI Lucknow in KBr phase.
Preparation of Ligand
It was prepared by mixing 3-acetyl-6-methyl-2H-pyran-2,4(3H) dione (0.168g, 1 m mole) in 20 ml methanol with furoic acid hydrazide (0.126 g, 1 m mole) in 20 ml methanol in 1 : 1 proportion and the mixture was refluxed and for 4 h. On cooling, a yellowish crystalline compound separated out. It was recrystallized from methanol. The purity of the schiff base was checked by TLC.
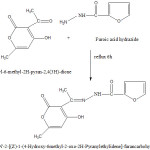 |
Scheme 1 Click here to View Scheme |
Preperation of Complexes
The ligand and the metal salts were mixed in the 2 : 1 molar ratio and kept overnight, when metal chelates separated out. The solid was filtered, washed with aqueous methanol and pet ether and drived in vacuum.
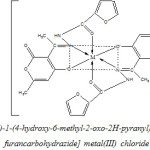 |
Scheme 2 Click here to View Scheme |
Results and Discussion
The analytical data of the metal chelates suggested 1:2 (M:L) stoichiometry for all the complexes and is in good agreement with the proposed molecular formulae and proposed structures of the complexes. All the metal complexes are colored and non hygroscopic. The Ti (III), & V (III) complexes are sensitive to air but all other complexes are stable in air. The complexes are insoluble in water and common organic solvents but soluble in DMF, DMSO & acetonitrile. The molar conductance values in DMF suggested 1 : 1 electrolytic nature for all the complexes.
The ir spectrum of the ligand exhibits bands at 3500, 1720, 1670, 1620 and 1310 cm–1 assignable to u OH(hydrogen bonded), u C=O(azide), u C=N (azomethine) and u C=O (phenolic) respectively. The uC–O–C of furan ring appeared at 1080 cm–1. In the spectra of the complexes u C–O lactone has undergone upward shift 25–30 cm–1 (15). The upward shift of u C=O (phenolic) in the spectra of the complex indicated the participated of phenolic oxygen in coordination (16). The azomethine frequency has undergone 20–25 cm–1 in the complexes suggesting coordination through N-atom of the azomethine group thus the ligand has behaved in mono basic tridentate manner. These coordination site as further supported by the presence of two non ligand bands in the ir spectra of the complex in the region of 540-560 cm–1 and 450–470 cm–1 assignable u M–O, u M–N frequencies respectively.
Electronic Spectra –
The magnetic susceptibility of Ti (III) complex was determined by Gouy’s method at room temperature which gave a value of 1.96 B.M. for magnetic moment. This value indicated presence of one unpaired electron. The higher value may be due to orbital contribution. The electronic spectrum of the complex shows a single broadband at 19430 cm–1 assignable to 2t2g ® 2Eg transition (17) which is characteristics of octahedral geometry.
The magnetic moment for Mn (III) complex is 4.91 mB revealing the high spin nature of the complex, corresponding to four unpaired electrons. Electronic spectrum shows a strong band at 20000 cm–1 which can be assigned to ligand to metal charge transfer and a shoulder at 18310 cm–1 which may be assigned to the 5E2g®5T2g transition (18), characteristic of octahedral geometry.
The Co (III) complex is diamagnetic (at 27°C) as expected for a low spin d6 ion. The electronic spectrum of cobalt (III) complex displayed bands at 15110, 15450, 21095–21640 and 23370–23860 cm–1. These are similar to these reported for other six coordinated cobalt (III) complexes and may be assigned to 1A1g ® 3T2g, 1A1g ® 1T1g and 1A1g ® 1T2g transitions, respectively (19).
The Fe (III) complex shows magnetic movement 5.20 mB which indicates high spin octahedral geometry corresponding to five unpaired electrons. The complex shows bands at 17666, 23825, 29880 cm–1 corresponding to 6Atg ® 4Ttg, 6Atg ® 4T2g and 6Atg ® 4Eg transition respectively. All these bands are characteristics of octahedral geometry.
The electronic spectrum of the Ru(III) complex displayed band at 18710, 22620 and 27630 cm–1 which has been assigned to 2T2g ® 4T1g, 2T2g ® 4T2g and 2T2g ® 2A2g, 2T1g transitions respectively. There assignment are comparable to these reported for other Ru(III) derivatives involving nitrogen oxygen donor molecules.
The magnetic moment of Cr (III) complex is 3.80 B.M., which suggests the presence of three unpaired electrons. The electronic spectrum showed three bands at 17750, 26200 and 37410 cm–1 which are assigned to 4A2g ® 4T2g (F), 4A2g ® 4T1g (F) and 4A2g ® 4T1g (P) transitions respectively for octahedral stereo chemistry.
Conclusion
On the basis of study performed and discussed Octahedral geometer has been propose a for all the synthesized M(III) complexes
Acknowledgment
The authors are thankful to the head chemistry department Dr. V.K. Sinha & Dr. S.K. Pant, Principal K.G.K. (P.G.) College, Moradabad for providing facilities to carry out the research work.
References
- Fontes, A.P.S.; Guerra, W.; Machado, F.C. Transition Met. Chem. 2004, 29, 382.
- Sharma, V.K.; Srivastava, S. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2005, 35,311.
- Iskander, M.F.; El-Sayed, L.; Salem, N.M.H.; Werner, R.; Haase, W. J. Coord. Chem. 2005,58, 125.
- Martsinko, E.E.; Seifullina, I.I.; Ya, Zub. V. Russ. J. Coord. Chem. 2005, 31, 795.
- Goudar, T.R.; Maravalli, P.B. Indian J. Chem. 1998, 37A, 83.
- Gudasi, K.B.; Goudar, T.R. Pol. J. Chem. 1999, 73, 385.
- Yan, W.; Zheng, Y.Y.; Bao, D. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2005, 35,237
- Rehman, S.U.; Mazhar, M.; Amin, B.; Sadiq, A.; Xuequing, S.; George, E.; Khalid, M.K. Synth. React. Inorg. Met. Org. Nano -Met. Chem. 2004, 34, 1379.
- Yan, W.; Zheng-yin, Y.; Bao-dui, W. Transition Met. Chem. 2005, 30, 879.
- Deepa, K.; Aravindakshan, K.K. Synth. React. Inorg. Met. Org . Nano-Met. Chem. 2005, 35,409.
- Chang, T.Y.; Ranford, J.D.; Jagdese, J.V. Synth. React. Inorg. Met. Org., Nano-Met. Chem.2005, 35, 71.
- Michael, D.; Malachy, Mc.C.; Dennis, O.; Rachel, K.; Denise, E.; Carol, D. J. Inorg. Biochem. 2004, 98, 1023.
- Salem, M.H.N. Synth. React. Inorg. Met. Org., Nano-Met. Chem. 2005, 35, 369.
- Venkateswar Rao, P.; Venkata Narasaiah, A. Indian J. Chem. 2003, 42A, 1896.
- Singh, B.; Singh, U.R. Transition Met. Chem. 1995, 20, 100.
- Mamatha, K.; Rupini, B.; Srihari. S. J. Indian Chem. Soc. 2004, 81, 950.
- Sanik, K Gupta, S. Roy, T.N. Mandal, K Das & S. Ray J. Cham. Sci, 122, 239 (2010).
- Rahul K. Rastogi, Poonam Garg & Shamim Ahmad, Asian J. Chem. 21, 6144 (2009)
- T. Daniel, Thavgadusai & K. Natrajan, Indian J. Chem. 41A, 741 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









