A Facile Microwave Assisted Synthesis and Spectral Analysis of 2-Amino-5-substituted phenyl-1,3,4-Oxadiazoles
Sanjeev Kumar1, Sneha Yadav1, Sudha Jadon1, Vipin Kumar1, Abdalla M. Khedr2 and Kishan C. Gupta*
1Department of Chemistry, Agra College, Agra - 282 002, India. 2Department of Chemistry, Faculty of Science, Tanta University, Tanta, Egypt. 3Former Director, Anand College of Pharmacy, Keetham, Agra - 282 007, India
Article Received on :
Article Accepted on :
Article Published : 22 Oct 2016
An efficient synthesis for the preparation of some 2-amino-5-substituted phenyl-1,3,4-oxadiazoles by using both conventional and microwave method have been devised. The obtained results revealed that, microwave assisted technique is efficient, eco-friendly and inexpensive method which not only give higher yield but also reduces the reaction time significantly. The resulting compounds were characterized by IR, 1H-NMR, UV-Vis and mass spectral analysis.
KEYWORDS:Oxadiazoles; Microwave assisted synthesis; Spectral analysis
Download this article as:| Copy the following to cite this article: Sanjeev Kumar S, Yadav S, Jadon S, Kumar V, Khedr A. M, Gupta K. C. A Facile Microwave Assisted Synthesis and Spectral Analysis of 2-Amino-5-substituted phenyl-1,3,4-Oxadiazoles. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Sanjeev Kumar S, Yadav S, Jadon S, Kumar V, Khedr A. M, Gupta K. C. A Facile Microwave Assisted Synthesis and Spectral Analysis of 2-Amino-5-substituted phenyl-1,3,4-Oxadiazoles. Available from: http://www.orientjchem.org/?p=22857 |
Introduction
Synthetic organic chemists explore new methods for chemical transformation. In recent year lot of work has been carried out to use microwave irradiation as an alternative to conventional heating which provide higher yield and cleaner product. 1,3,4-oxadiazole belongs to an important class of heterocyclic compounds possess wide spectrum of pharmacological, medicinal and biological activities. For example 2-amino-1,3,4-oxadiazoles act as muscle relaxant1 and show antimitotic2, antibacterial3, anti-inflammatory4, anticonvulsant5, CNS stimulant6, hypertensive7, antimicrobial8, insecticidal9, anticancer10, antituberculostic11, antiviral12, antiparkinsonian13, antiproliferative14, antiprotozoal15, analgesic16, antihistaminic17 activities. They are also used for material research for preparation of organic light emitting diode, photoluminescence polymers etc18-20. Keeping the above fact in view we report facile synthesis of 2-amino-5-substituted phenyl-1,3,4-oxadiazoles using the microwave and conventional methods. The IR, 1H-NMR, UV-Vis and mass spectra of the synthesized oxadiazoles are investigated and discussed in relation to their molecular structures.
Experimental
Materials and Methods
All the aldehydes semicarbazide and anhydrous sodium acetate of AR grade and E. Merck make were used in all the reactions. Melting points were determined by open capillary method using the ‘Tempo’ make melting point apparatus and are uncorrected. The purity and homogeneity of compounds as well as completion of reaction time was checked by thin layer chromatography (TLC) using silica gel-G as adsorbent and solvent system used benzene:chloroform:methanol (5:4:1). The spots were visualized by iodine vapours after irradiation with UV light. All the compounds were purified by preparative TLC/Column chromatography. IR spectra were recorded in KBr discs on a Perkin–Elmer (model 1430) IR spectrometer (4000-400 cm-1) at the Micro-analytical unit of Tanta University, Egypt. 1H-NMR were measured on a Bruker DMX 750 (500 MHz) FT spectrometer using d6 DMSO as a solvent. UV-Vis spectra were carried out using a Cary-400 double beam recording spectrophotometer within the wavelength range 190-700 nm at room temperature. Electron spray ionization (ESI) mass spectra were taken on a Shimadzu LCMS-2010 eV mass spectrometer at the GakushuinUniversity (Japan).
General procedure for the preparation of 1,3,4-oxadiazoles (Sa-Sf)
A) Conventional method
To a stirred solution of substituted semicarbazone (0.01 mole), 0.02 mole of anhydrous sodium acetate in 25 ml glacial acetic acid and a solution of bromine (0.7 ml in 5 ml glacial acetic acid) were added drop wise at room temperature. The solution was stirred for 25-30 minutes and then added to ice cold water. The precipitated product was collected by filtration and washed with water. The product was dried and recrystallised from ethanol.
B) Microwave method
Substituted semicarbazone (0.01 mole) and sodium acetate (0.02 mole) were dissolved in 25 ml of glacial acetic acid. The reaction mixture was transferred to conical flask fitted with guard tube. A solution of bromine (0.7 ml in 5 ml glacial acetic acid) was added drop wise at room temperature. The reaction mixture was heated for 15-20 seconds at 480 watt microwave power. The progress of reaction was monitored by TLC (silica gel–G) using benzene:chloroform:methanol (5:4:1) as solvent system. The reaction mixture was cooled at room temperature and poured on crushed ice, the product was precipitate out, filtered, washed with water, dried and recrystallised from ethanol.
Results and Discussion
The oxadiazoles (Sa-Sf) were prepared using Scheme-1. The melting points, reaction time, yield and other physical data of synthesized oxadiazoles are given in Table-1, while spectral data are discussed in Table-2.
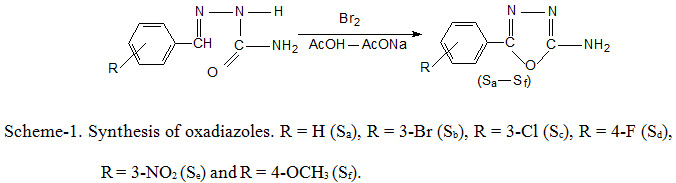
The data in Table-1 confirm that there was significant difference in time taking for the preparation of oxadiazoles by conventional method which ranges from 25-30 minutes. While oxadiazoles preparation using microwave method takes only 15-20 seconds for completion of reaction. Also, there was significant difference in yield of the oxadiazoles prepared by conventional and microwave assisted synthesis. Conventional method gave poor yield of oxadiazoles ranging from 60.1-74.1%, while microwave method provide better yield ranging between 77.6-92.1%. These data revealed that microwave assisted technique is efficient, eco-friendly, and inexpensive method which not only give higher yield but also reduces the reaction time significantly.
Table 1: Physical Characteristics of Synthesized Oxadiazoles (Sa-Sf).
|
S |
R |
Molecular formula |
Molecular weight |
M.P. (oC) |
Conventional method |
Microwave method |
||
|
Time (min) |
Yield (%) |
Time (sec) |
Yield (%) |
|||||
|
Sa |
H |
C8H7N3O |
161.1628 |
242 |
25 |
68.2 |
15 |
77.6 |
|
Sb |
3-Br |
C8H6N3OBr |
240.0589 |
239 |
30 |
62.2 |
20 |
89.0 |
|
Sc |
3-Cl |
C8H6N3OCl |
195.6079 |
153 |
30 |
69.1 |
20 |
77.7 |
|
Sd |
4-F |
C8H6N3OF |
179.1533 |
230 |
25 |
60.1 |
15 |
79.0 |
|
Se |
3-NO2 |
C8H6N4O3 |
206.1604 |
262 |
30 |
74.1 |
20 |
92.1 |
|
Sf |
4-OCH3 |
C9H9N3O2 |
191.1890 |
248 |
25 |
67.2 |
15 |
83.1 |
Formation of oxadiazoles (Sa-Sf) was confirmed by IR spectral data (Table-2). The appearance of strong absorption bands at 925-975 and 1020-1050 cm-1 range is due to C-O-C linkage of the oxadiazoles ring. All compounds displayed strong bands at 1620-1665 cm-1 range corresponding to C=N stretching frequencies21. Beside the above characteristic band, all the compounds showed a doublet in the region 3032-3412 cm-1 corresponding to the symmetric stretching vibration of –NH2 group. The stretching vibrations of the aromatic C-H groups give medium to weak bands within the 2768-2784 cm-1 range, while the bands appeared at 832-917 cm-1 are assigned to n(Ar-H stretch).
Table 2: UV-Vis, IR and 1H-NMR Spectral Data of Synthesized Oxadiazoles (Sa-Sf).
|
S |
UV-Vis (λmax, nm) |
IR (KBr cm-1) |
1H-NMR (DMSO-d6, δ ppm) |
|
Sa |
206, 275 |
974, 1027 (C-O-C oxadiazole ring), 1652 (C=N strech), 3116-3294 (-NH2), 2782 (Ar-CH), 917 (Ar-H). | 7.50-7.80 (m, 5H phenyl), 7.37 (s, 2H, NH2) |
|
Sb |
207, 282 |
966, 1044 (C-O-C oxadiazole ring), 1652 (C=N strech), 3110-3295 (-NH2), 2768 (Ar-CH), 895 (Ar-H). | 7.66-7.87 (m, 3H phenyl), 7.47 (m, 1H), 7.36 (s, 2H, NH2). |
|
Sc |
213, 284 |
931, 1048 (C-O-C oxadiazole ring), 1656 (C=N stretch),3116-3291 (-NH2), 2784 (Ar-CH), 891 (Ar-H). | 7.73-7.92 (m, 3H, 4, 5, 6 phenyl), 7.58 (s, 1H, 2phenyl), 7.36 (s, 2H, NH2). |
|
Sd |
206, 274 |
971, 1027 (C-O-C oxadiazole ring), 1652 (C=N stretch), 3113-3295 (-NH2), 2780 (Ar-CH), 917 (Ar-H). | 7.51-7.77 (m, 2H, 4, 5, 6 phenyl), 7.51-7.77 (m, 2H phenyl), 7.26 (s, 2H, NH2). |
|
Se |
208, 270 |
928, 1047 (C-O-C oxadiazole ring), 1665 (C=N stretch), 3032-3412 (-NH2), 2780 (Ar-CH), 890 (Ar-H). | 8.22-8.49 (m, 3H phenyl), 7.83 (m, 1H phenyl), 7.43 (s, 2H, NH2). |
|
Sf |
207, 281 |
971, 1022 (C-O-C oxadiazole ring), 1658 (C=N stretch), 3130-3318 (-NH2), 2769 (Ar-CH), 832 (Ar-H). | 7.09-7.71 (m, 2H phenyl), 7.09-7.77 (m, 2H phenyl), 7.05 (s, 2H, NH2), 3.79 (S, 3H OCH3). |
The structures further confirmed by 1H-NMR, where multiplet peaks for aromatic protons in the region of 7.09-8.49 ppm were observed22. The methoxy proton was observed as singlet at 3.79 ppm, while amino proton was observed as singlet in the region 7.05-7.43 ppm. The signals observed in the 1H-NMR spectra of the prepared oxadiazoles and their assignments are collected in Table-2.
The electronic absorption spectra of the oxadiazoles were scanned in methanol (200-500 nm) and displayed two main bands (Table-2). The first band located within 206-213 nm range is attributed to the high energy p—p* transition corresponding to the 1La—1A state in the aromatic moiety22. The second band at 270-284 nm range is due to n—p* transition in the CH=N group23.
The structure of synthesized 2,5-disubstituted-1,3,4-oxadiazoles were also confirmed by mass spectral analysis. Under EI condition, Sa and Sb showed prominent molecular ion peaks at m/z 162.21 and 241.31 corresponding to M++1. The prominent mass peaks appeared at m/z 195.60, 179.00, 206.00 and 191.10 in the mass spectra of Sc, Sd, Se and Sf are due to the molecular weight of the parent ion [M] +. All the above data confirms the TLC and spectroscopic purity of the prepared oxadiazoles (Sa-Sf).
Conclusion
It can be concluded that microwave assisted synthesis is simple, inexpensive, fast and facile in comparison to previously reported conventional methods in reducing the heating time and improving the yield of the clean product.
Acknowledgments
The authors express their sincere thanks to Dr. Mohamed El-Zarie (Gakushuin University, Japan) for performing the 1H-NMR and mass spectral analysis.
References
- Yale H.L. and Losee K.J., J. Med. Chem., 9, 478 (1966).
- Ghirian D., Schwatz L. and Simiti I., Farmacia, 22, 141 (1974).
- Mishra B.A. and Nizammudin J., J. Indian Chem. Soc., 27, 5576 (1998).
- Ramalingam T., Deshmukh A.A., Sattur P.B., Sheth U.K. and Naik S.R., J. Indian. Chem. Soc., 26, 58 (1981).
- Ram Y.J. and Pandeya H.N., J. Indian. Chem. Soc., 51, 634 (1974).
- Dubey A.K. and Sangwan N. K., Indian. J. Chem., 33B, 1043 (1994).
- Ponticello G.S., Engelhardt E.L. and Baldwin J.J., Indian J. Heterocycl. Chem., 17, 425 (1980).
- Shawali A.S., Abudllah M.A. and Zayed M.E.M., Z. Naturforsch B, 55, 546 (2000).
- Mohan T.P., Vishalakshi B., Bhat K.S., Rao K.S. and Kendappa G.N., Indian. J. Chem. Soc., 48B, 1798 (2004).
- Holla B., Prasnna S.C.S., Boja P., Rao K.S., Bhatt S. and Ganesh U., Indian J. Chem., 43B, 864 (2003).
- Silesirni B. and Pagatili C., J. Phycol., 16, 209 (1961).
- Yadav L.D.S. and Singh S., Indian J. Chem., 40B, 440 (2001).
- Hazarika J. and Kataky J.C., Indian J. Heterocycl. Chem., 8, 83 (1998).
- Liszkieicz H., Kowalska M.W., Wietrzyk J. and Opolski A., Indian J. Chem., 42B, 2846 (2003).
- Weston J.B., Eur. Pat., 7529 (1980).
- Ram V.J., Indian J. Chem., 27B, 825 (1998).
- Ajitha M., Rajnarayana K. and Sangarapani K., Pharmazie, 57, 796 (2002).
- Cheng D., Ma F. and Liu X., Opt. Laser Technol., 39, 720 (2007).
- Quan S., Tang F., Xu Z., Qian L., Zhang T., Liu D., Hou Y., Wang Y. and Xu X., J. Luminescence, 124, 81 (2007).
- Tang H., Song N., Gao Z., Chen X., Fan X., Xiang Q. and Zhou Q., Polym. J., 48, 129 (2007).
- Chandra S. and Gupta L.K., Spectrochim. Acta A, 62, 1089 (2005).
- Issa R.M., Khedr A.M. and Rizk H., J. Chin. Chem. Soc., 55, 875 (2008).
- Moustafa M.E., El-Mossalamy E.H. and Amin A.S., Monatsh. Chem., 126, 901 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









