Vanadatesulfuric Acid-Catalyzed Novel And Eco-Benign One-Pot Synthesis Of Polyhydroquinoline Derivatives Under Solvent-Free Conditions
Masoud Nasr-Esfahani* and Tooba Abdizadeh
Department of Chemistry, Yasouj University, Yasouj, Iran
A novel and green approach for efficient and rapid synthesis of biologically active polyhydroquinoline derivatives via unsymmetric Hantzsch reaction using Vanadatesulfuric acid (VSA) as a new and recyclable catalyst under solvent-free conditions was reported. The catalyst was characterized by FT-IR, XRD and XRF analysis. The present method offers several advantages such as simple procedure, short reaction time, high yields, simple workup, reusability of the catalyst and simple purification of the products.
KEYWORDS:aldehyde; 1;3-cyclohexanediones; Heterogeneous catalysis; Polyhydroquinoline; Vanadatesulfuric acid
Download this article as:| Copy the following to cite this article: Nasr-Esfahani M, Abdizadeh T. Vanadatesulfuric Acid-Catalyzed Novel And Eco-Benign One-Pot Synthesis Of Polyhydroquinoline Derivatives Under Solvent-Free Conditions. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Nasr-Esfahani M, Abdizadeh T. Vanadatesulfuric Acid-Catalyzed Novel And Eco-Benign One-Pot Synthesis Of Polyhydroquinoline Derivatives Under Solvent-Free Conditions. Available from: http://www.orientjchem.org/?p=22957 |
Introduction
In pharmaceutical view, heterocycles are of superior importance in the discovery and design of new compounds1. Recently, an increasing attention has been focused toward the multicomponent synthesis of 1,4-dihydropyridyl compounds due to their broad range of biological activities2. 1,4-dihydropyridines as analogues of NADH coenzymes, such as nifedipine, nicardipine, amlodipine, and other related derivatives, are widely used as calcium channel blockers for the treatment of cardiovascular disorder including angina, hypertension and cardiac arrhythmias3. 1,4-Dihydropyridines are calcium antagonists4, antitubercular agents5, and neuropeptide Y Y1 receptor antagonists6. They possess neuroprotective7, platelet antiaggregation8, antidiabetic activities9 cerebral antischemic activity in the treatment of Alzheimer’s disease, and chemosensitizer in tumor therapy10. Also quinolines are very important compounds. Members of this group are being used as anti-inflammatory, antimalarial, antibacterial, antiasthamatic, and tyrosine kinase inhibiting agents11.
These examples clearly demonstrate the remarkable potential of novel DHP family as a source of valuable drug candidates. Thus, the synthesis of this heterocyclic nucleus is of great importance.
More than a century ago the synthesis of 1,4-dihydropyridines by classical Hantzsch method12, a one-pot condensation of an aldehyde with alkyl acetoacetate and ammonia, was presented. However, the yields of these compounds obtained by the Hantzsch synthesis are generally low.
Therefore, numerous promotions, such as using ionic liquids13,14, microwaves15-17, grinding18, silica-supported acids19,20, L-proline21, HY-zeolite22, Bu4NHSO423, boronic acids24,25, TMSCl–NaI26, ceric ammonium nitrate27,28, metal triflates29,30, p-TSA31, and baker’s yeast32,33 have been developed. However, many of these methods still suffer from several limitations, such as unsatisfactory yield, use of environmentally suspected organic solvents, long reaction time, high temperature, and using expensive and non reusable catalysts. Therefore, investigation for improved reaction conditions for synthesis of polyhydroquinolines using novel and reusable catalysts under solvent-free conditions is still desired.
Multicomponent reactions as an efficient and dominant tool in modern synthetic organic chemistry allow the facile creation of several new bonds in a one-pot reaction. This reduces the reaction time, and saves money, energy, and raw materials34. Therefore, research in industry and academic has increasingly emphasized the use of multicomponent reactions as well as domino reaction sequences for a wide range of products35.
Solid acid catalysts with lower toxicity, higher stability and recyclability play a prominent role in organic synthesis under heterogeneous conditions. Solid acids have many advantages such as ease of handling, decreasing reactor and plant corrosion problems, and environmentally safe disposal36,37.
It is well known in the protocol of green chemistry that its main objective is to perform reactions under solventless conditions using heterogeneous catalysts, in order to generate environmentally friendly chemical transformations38. In addition, it is important to note that an ideal synthesis is considered as one in which a target molecule is produced quantitatively in one step, from available and inexpensive raw materials, under environmentally harmless processes39.
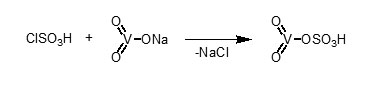
In continuation of above and our studies on the application of inorganic solid acid40, we found that anhydrous sodium metavanadate reacts with chlorosulfonic acid (1:1 mole ratio) to give vanadatesulfuric acid (VSA). The reaction is performed easy, clean and without any workup (Scheme 1).
Experimental
Chemicals were purchased from Merck, Fluka and Aldrich chemical companies. X-ray diffraction analysis was carried out using a D8 ADVANCE, Bruker X-ray diffractometer using Cu-Kα radiation (λ = 1.5406 A˚). Melting points were determined using a Barnstead Electrothermal (BI 9300) apparatus and are uncorrected. IR spectra were obtained using a FT-IR JASCO-680 spectrometer instrument. NMR spectra were taken with a Bruker 400 MHz Ultrashield spectrometer at 400MHz (1H) and 125 MHz (13C) using CDCl3 or DMSO-d6 as the solvent with TMS as the internal standard.
Preparation of vanadatesulfuric acid
Anhydrous sodium metavanadate was prepared by drying of sodium metavanadate. monohydrate (NaVO3. H2O, MW = 139.94) in the oven at 250 °C for 4 hours. To 0.1 mol of chlorosulfonic acid (11.6 g, 7.7 mL) in 250 mL round bottom flask in the ice-bath, 0.1 mol (12.2 g) anhydrous sodium metavanadate was added gradually with stirring. After the completion of addition of anhydrous sodium metavanadate, the reaction mixture was shaken for 1 h. Then 50 mL of cold water was added to the reaction mixture and stirred for 10 minutes. The mixture was filtered and a dark red solid of vanadatesulfuric acid, 16.3 g (91%), Mp 256 °C (dec.) was obtained. Characteristic IR bands (KBr, cm-1): 3540-3300 (OH, bs), 1640 (OH, m), 1250-1140 (S=O, bs), 1050 (S-O, m), 960 (V=O, m), 840 (V=O, m), 630 (V-O, m).
General procedure for the preparation of polyhydroquinolines
In a round-bottomed flask the aldehyde (1mmol), 1,3-cyclohexanedione derivatives (1 mmol), ammonium acetate (1.5 mmol), β-ketoester (1 mmol) and VSA (10 mol%) were mixed thoroughly. The flask was heated at 80 °C with concomitant stirring. After completion of the reaction confirmed by TLC (eluent: EtOAc:n-hexane, 1:4), hot ethanol (10 mL) was added and filtered and separated solid catalyst. The solvent was evaporated and the crude products were recrystallized from ethanol, gave the pure products in 80-95% yields based on the starting aldehyde (Table 2). The products were characterized by IR, 1H NMR, 13C NMR and via comparison of their melting points with the reported ones. Spectroscopic data of new compounds:
2,7,7-Trimethyl-5-oxo-4-(4-nitrophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid methyl ester (Table 2, entry 23)
Mp: 251-253 °C; Rf= 0.53 (n-hexane:ethyl acetate = 4:1) ; IR (KBr): 3275, 3189, 3073, 2968, 1709, 1606, 1516, 1430, 1376, 1216, 1074, 864, 721 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.93 (s, 3H), 1.11 (s, 3H), 2.21-2.38 (m, 4H), 2.45 (s, 3H), 3.62 (s, 3H), 5.18 (s, 1H), 6.12 (brs, 1H), 7.48 (d, J = 8.4 Hz, 2H), 8.10 (d, J = 8.4 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 18.35, 18.52, 26.35, 28.99, 32.11, 36.38, 50.00, 50.78, 102.02, 109.00, 123.22, 128.55, 145.62, 146.40, 150.08, 154.78, 166.86, 194.25.
2,7,7-Trimethyl-5-oxo-4-(4-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid methyl ester (Table 2, entry 24)
Mp: 259-261 °C; Rf= 0.55 (n-hexane:ethyl acetate = 4:1); IR (KBr): 3274, 3184, 3070, 2954, 1704, 1605, 1496, 1428, 1378, 1213, 1070, 846, 778 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.95 (s, 3H), 1.09 (s, 3H), 2.24-2.72 (m, 2H), 2.38 (s, 2H), 2.42 (s, 3H), 3.63 (s, 3H), 3.75 (s, 3H), 5.02 (s, 1H), 6.48 (brs, 1H), 6.75 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.8 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 18.26, 18.53, 26.42, 29.12, 32.10, 34.66, 50.61, 54.80, 103.47, 110.19, 113.13, 128.18, 139.80, 144.95, 149.19, 157.24, 167.37, 194.29.
2,7,7-Trimethyl-5-oxo-4-(3-nitrophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid methyl ester (Table 2, entry 25)
Mp: 234-236 °C; Rf= 0.53 (n-hexane:ethyl acetate = 4:1) ; IR (KBr): 3298, 3077, 2975, 1709, 1700, 1606, 1482, 1427, 1377, 1221, 1073, 874, 688 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.95 (s, 3H), 1.11 (s, 3H), 2.18-2.45 (m, 4H), 2.44 (s, 3H), 3.64 (s, 3H), 5.18 (s, 1H), 6.3 (brs, 1H), 7.38-7.42 (t, J = 8.0 Hz, 2H), 7.74 (d, J = 7.6 Hz, 1H), 7.99-8.01 (d, J = 6.0 Hz, 1H), 8.01 (d, J = 6.0 Hz, 1H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 18.38, 18.52, 26.23 , 29.04, 32.17, 36.10, 49.97, 50.82 , 102.24, 109.19, 120.96, 121.68, 129.46, 134.14, 146.36, 147.49, 149.48, 150.11,166.90, 194.35.
2,7,7-Trimethyl-5-oxo-4-(4-bromophenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid methyl ester (Table 2, entry 26)
Mp: 263-264 °C; Rf= 0.46 (n-hexane:ethyl acetate = 4:1) ; IR (KBr): 3288, 3198, 3076, 2957, 1682, 1605, 1491, 1379, 1225, 1073, 837, 774, 537 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.86 (s, 3H), 1.01 (s, 3H), 2.13-2.21 (m, 4H), 2.33 (s, 3H), 3.54 (s, 3H), 4.95 (s, 1H), 6.22 (brs, 1H), 7.09 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 18.30, 18.53, 26.37, 29.05, 32.11, 50.10, 50.71, 102.64, 109.57, 118.73, 129.55, 130.41, 145.70, 146.78, 149.61, 167.10, 194.28.
2-Propyl-7,7-dimethyl-5-oxo-4-(4-methoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (Table 2, entry 27):
Mp: 191-193 °C; Rf= 0.59 (n-hexane:ethyl acetate = 4:1) ; IR (KBr): 3278, 3213, 3086, 2959, 1698, 1606, 1490, 1380, 1211,1084, 851, 758 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.95 (s, 3H), 1.03 (t, J = 7.2 Hz, 3H), 1.08 (s, 3H), 1.23 (t, J = 7.2 Hz, 3H), 1.68 (q, J = 6.5 Hz, 2H), 2.16-2.38 (m, 4H), 2.68-2.82 (m, 2H), 3.75 (s, 3H), 4.07(q, J = 7.2 Hz, 2H), 5.02 (s, 1H), 6.3 (brs, 1H), 6.74 (d, J = 4.8 Hz), 7.12 (d, J = 2.4 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 13.71 , 14.08 , 21.87, 26.39, 29.15, 31.90, 32.83, 34.87, 50.21, 54.77, 59.00, 103.64, 109.97, 113.02, 128.29, 139.99, 148.76, 149.41, 157.22, 166.61, 194.17.
2-Propyl-7,7-dimethyl-5-oxo-4-(4-chlorophenyl)-1,4,5,6,7,8-hexahydroquinoline-3- carboxylic acid ethyl ester (Table 2, entry 28)
Mp: 219-221 °C; Rf= 0.54 (n-hexane:ethyl acetate = 4:1); IR (KBr): 3275, 3210, 3086, 2961, 1703, 1609, 1489, 1380, 1211, 1084, 843, 770 cm-1; 1H NMR (400 MHz, CDCl3) d (ppm): 0.93 (s, 3H), 1.05 (t, J = 7.2Hz, 3H), 1.19 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H), 1.68 (m, 2H), 2.21 (d, J = 16.4 Hz, 2H), 2.29 (d, J = 16.2 Hz, 2H), 2.40 (d, J = 15.6 Hz, 2H), 2.69-2.84 (m, 2H), 4.07 (q, J = 7.2 Hz, 2H), 5.05 (s, 1H), 6.32 (brs, 1H), 7.18 (d, J = 8.8 Hz, 2H), 7.24 (d, J = 8.8 Hz, 2H); 13C NMR (400 MHz, DMSO-d6) d (ppm): 13.72, 14.04, 18.53, 21.87, 26.33, 29.10, 32.10, 32.83, 35.53, 50.09, 59.13, 102.87, 109.40, 127.68, 129.03, 130.16, 146.56, 149.51, 149.82, 166.33, 194.17.
Results and discussion
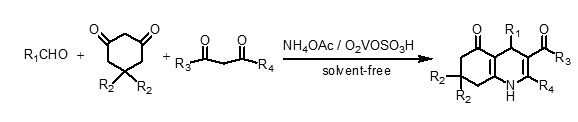
In this paper, we wish to report a novel, mild, cost-effective, and environmentally benign procedure for the one-pot synthesis of polyhydroquinoline derivatives by the condensation of aldehydes (aromatic, aliphatic, unsaturated, and heterocyclic), 5,5-dimethyl-1,3-cyclohexanedione (dimedone) or 1,3-cyclohexanedione, β-ketoesters and ammonium acetate in the presence of a catalytic amount of vanadatesulfuric acid (VSA) under solvent-free conditions (Scheme 2).
Characterization of vanadatesulfuric acid
Figure 1 exhibits the IR spectra of sodium metavanadate as substrate and Vanadatesulfuric acid. The infrared vibration bands found for NaVO3 are assigned as follows (Fig 1(a)): In these spectra, several absorptions appear which are apparently the result of V-O stretching modes for each of several, different oxygen atoms according to the particular location or arrangement within the lattice40.At lower frequency, a broad and general absorption occurs which is apparently caused by lower frequency VO bonding. Here, the V-O stretching mode is observed as a medium band located at 950 cm-1. These two spectra tend to locate the “normal” position for this stretching vibration between oxygen and vanadium. Other broad bands are present in the spectrum of sodium metavanadate, centering at 845 and 690 cm-1. The VO3– structure consists of VO bondings of variable bond lengths, some of which vibrate at lower frequencies than others. The 950 cm-l band has been assigned to a VO bond which is considerably shorter than other bonds in the structure; the 845 cm-1 band very probably arises from the stretching modes of the longer VO bonds. For Vanadatesulfuric acid, the infrared vibration bands are consigned as follows (Fig. 1(b)): The bands found at 3450 and 1640 cm-1 are attributed to the stretching and bending vibration of -OH group, respectively. The bands at 1050, and 1180 cm-1 are assigned for the sulfonic acid bonds, S–OH, S=O stretching, and S=O asymmetric stretching, respectively. The bands appearance in 960, 840 and 603 cm-1 related to V=O and V-O stretching.
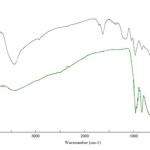 |
Figure 1 : FT-IR spectra of (a) sodium metavanadate; (b) vanadatesulfuric acid. Click here to View figure |
Figure 2 depicts the X-ray diffraction (XRD) pattern of VSA. A number of prominent Bragg reflections reveal that the resultant particles of Vanadatesulfuric acid have a monoclinic structure (Space group: P2/m; a = 12.170 A°, b = 3.602 A°, c = 7.780 A°, JCPDS card no. 16-0601). The size of the VSA particles was also determined from X-ray line broadening using the Debye-Scherrer formula (D = 0.9λ/βcosθ, where D is the average crystalline size, λ is the X-ray wavelength used, β is the angular line width at half maximum intensity, and θ is the Bragg’s angle). For the (001) reflection the average size of the VSA particles was estimated to be around 16 nm. In addition, elemental analysis of catalyst was performed by means of X-ray fluorescence analysis (XRF) that the obtain result confirmed the elemental composition of VSA.
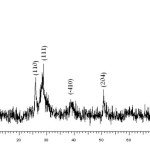 |
Figure 2. Powder X-ray diffraction pattern of the VSA particles. Click here to View figure |
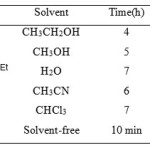 |
Table 1: Solvent effect on the model reaction catalyzed by VSA Click here to View table |
Effect of solvent and catalyst concentration on the synthesis of polyhydroquinolines
In order to get the best experimental conditions, we initially studied the effect of various solvents and catalytic efficiency of VSA for the synthesis of 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester (Table 2, entry 9) using the model reaction of benzaldehyde (1mmol), dimedone (1 mmol), ethyl acetoacetate (1mmol), and ammonium acetate (1.5 mmol). As shown in Table 1, among the tested solvents, such as ethanol, methanol, water, acetonitrile and a solvent-free system, the best result was obtained after 10 min under solvent-free conditions in excellent yield (95%).
Figure 3 illustrates the effect of catalyst molar ratio on the conversion time of benzaldehyde as typical substrate under solvent-free conditions. It is important to note that no polyhydroquinoline derivatives were afforded when the reactions were performed in the absence of VSA in the reaction mixture. With increasing the catalyst, the reaction time is decreased up to 10% of catalyst molar ratio that was found to be an optimum amount in current conditions. The higher amount of catalyst was found that have not a notable effect on the reaction time and yield.
<<Fig. 3>>
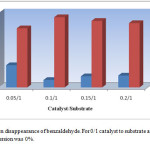 |
Figure 3: The catalyst amount effect on the synthesis of polyhydroquinolinesa |
Therefore, this reaction was developed with other aldehydes, and the results are summarized in Table 2. The time of reaction was within 5-45 min, and high yields of polyhydroquinolines were obtained.
Table 2. Synthesis of polyhydroquinoline derivatives in presence of VSA as catalysta
| Entry | R1 | R2 | R3 | R4 | Time(min) | Yieldsb(%) | Mp(˚C) | |
| Found | Reported | |||||||
| 1 | C6H5 | H | OEt | Me | 12 | 90 | 241-242 | 240-241 |
| 2 | 4-NO2C6H4 | H | OEt | Me | 14 | 86 | 205-206 | 204-205 |
| 3 | 4-CH3C6H4 | H | OEt | Me | 35 | 81 | 240-242 | 241-243 |
| 4 | 4-OHC6H4 | H | OEt | Me | 16 | 80 | 234-236 | 234-235 |
| 5 | 4-ClC6H4 | H | OEt | Me | 15 | 90 | 235-237 | 234-235 |
| 6 | 4-BrC6H4 | H | OEt | Me | 30 | 89 | 253-254 | 253-255 |
| 7 | 3-NO2C6H4 | H | OEt | Me | 15 | 92 | 201-203 | 200-201 |
| 8 | 2-NO2C6H4 | H | OEt | Me | 24 | 90 | 191-193 | 191-192 |
| 9 | C6H5 | Me | OEt | Me | 10 | 95 | 202-204 | 203-204 |
| 10 | 4-NO2C6H4 | Me | OEt | Me | 13 | 92 | 205-206 | 204-205 |
| 11 | 2-NO2C6H4 | Me | OEt | Me | 21 | 90 | 205-207 | 206-207 |
| 12 | 2-OCH3C6H4 | Me | OEt | Me | 12 | 85 | 193-195 | 193-195 |
| 13 | 3-BrC6H4 | Me | OEt | Me | 15 | 88 | 233-235 | 234-236 |
| 14 | 4-CH3C6H4 | Me | OEt | Me | 25 | 87 | 260-262 | 261-263 |
| 15 | 4-BrC6H4 | Me | OEt | Me | 10 | 91 | 252-254 | 253-255 |
| 16 | 3-NO2C6H4 | Me | OEt | Me | 10 | 93 | 179-181 | 178-180 |
| 17 | 2,4-Cl2C6H3 | Me | OEt | Me | 25 | 92 | 242-244 | 240-243 |
| 18 | CH3CH2CH2 | Me | OEt | Me | 45 | 78 | 147-149 | 147-148 |
| 19 | 2-Furyl | Me | OEt | Me | 10 | 80 | 247-248 | 246-248 |
| 20 | C6H5–CH=CH | Me | OEt | Me | 20 | 85 | 204-206 | 205-207 |
| 21 | 4-CH3C6H4 | Me | OMe | Me | 20 | 85 | 274-276 | >270 |
| 22 | 4-ClC6H4 | Me | OMe | Me | 5 | 91 | 219-221 | 220-222 |
| 23 | 4-NO2C6H4 | Me | OMe | Me | 10 | 92 | 251-253 | – |
| 24 | 4-CH3OC6H4 | Me | OMe | Me | 35 | 82 | 259-261 | – |
| 25 | 3-NO2C6H4 | Me | OMe | Me | 30 | 87 | 234-236 | – |
| 26 | 4-BrC6H4 | Me | OMe | Me | 25 | 89 | 263-264 | – |
| 27 | 4-CH3OC6H4 | Me | OEt | Pr | 35 | 85 | 191-193 | – |
| 28 | 4-ClC6H4 | Me | OEt | Pr | 20 | 89 | 219-221 | – |
aAll products were characterized by 1H NMR, 13C NMR and IR spectroscopy and comparison with these reported in the literature27,29,33,42.
b Isolated yields.
By using this heterogeneous catalyst, the aromatic aldehydes, bearing electron-donating substituents such as methyl, methoxy, and hydroxy and electron-withdrawing groups such as nitro and halid, gave high yields. The procedure worked well for vinyl as well as heterocyclic aldehydes in addition to aromatic aldehydes (Table 2). Acid-sensitive substrates such as cinnamaldehyde proceeded well to give the corresponding polyhydroquinoline without any side products (Entry 20). The results indicate the generality of the procedure, because aliphatic, aromatic, heterocyclic and α,β-unsaturated aldehydes were converted into the corresponding products in good to excellent yields in short reaction time as compared with some reported methods (Table 4).
To use of VSA in large scale synthesis especially in chemical laboratory, a typical reaction was performed for synthesis of 9 with tenfold amounts of reactants and catalyst with respect to one mentioned in the experimental section. The results showed the yield of 92% in these conditions that is comparable with one in table 2.
The reusability of the catalysts is an important benefit and makes them useful for commercial applications. Thus, the recovery and reusability of Vanadatesulfuric acid were investigated. The recyclability of the catalyst in the model reaction was checked. To achieve the reaction efficiency of recovered catalyst, the reaction mixture of 9 was filtered and washed with ethanol twice to give vanadatesulfuric acid. The recovered acid was dried and used again for synthesis of 9 that led to the yield of 90%. It can also be recovered and reused at least four times without any considerable loss of its activity (Table 3).
Table 3: Reusability of vanadatesulfuric acid in the synthesis of 2,7,7-trimethyl-5-oxo-4-phenyl-1,4,5,6,7,8-hexahydroquinoline-3-carboxylic acid ethyl ester
|
Cycles |
Isolated yield a ( %) |
| Fresh | 95 |
| 1 | 92 |
| 2 | 90 |
| 3 | 87 |
| 4 |
85 |
aCatalyst could be recycled by washing with ethanol and dried at 100 °C for 2h
Comparative results
In order to show the ability of our method with respect to previous reports, some of our results in comparison to some other methods are summarized in table 4. As shown, the yield/time ratio of the present method is better or comparable with the other reported results.
Table 4. Comparison of efficiency of various catalysts in the model reaction
| Entry | Catalyst | conditions | Time(h) | Yielda | Ref |
| 1 | ceric ammonium nitrate | Solvent-free/rt | 1 | 92 | 27 |
| 2 | Yb(OTf)3 | Ethanol/rt | 5 | 90 | 29 |
| 3 | baker’s yeast | phosphate buffer/rt | 24 | 79 | 32 |
| 4 | p-TSA | MeOH /reflux | 2.5 | 90 | 31 |
| 5 | Hf(NPf2)4 | Solvent free/60 0C | 3 | 95 | 43 |
| 6 | H3PW12O40 | Solvent-free/80 0C | 2 | 90 | 44 |
| 7 | GuHCl | Solvent-free/rt | 3 | 98 | 45 |
| 8 | this work | Solvent-free/80 0C | 10 min | 95 | – |
a Isolated yields
Conclusion
In summary, a novel and highly efficient method for the synthesis of polyhydroquinolines has been achieved by the multi-component condensation reaction of aldehydes (aromatic, aliphatic, unsaturated, and heterocyclic), 1,3-cyclohexanedione derivatives, ammonium acetate and β-ketoester using catalytic amount of the reusable and environmentally benign vanadatesulfuric acid (VSA) as a new solid acid catalyst under solvent-free conditions. The attractive features of this protocol are simple procedure, short reaction times, high yields, simple workup, reusability of the catalyst and simple purification of the products. Furthermore, this method is also expected to find application in organic synthesis due to the low cost of the catalyst. This approach could make a valuable contribution to the existing processes in the field of polyhydroquinolines synthesis.
Acknowledgement
We are thankful to Yasouj University for partial support of this work.
References
- Couladourous, E.A. and Strongilos, A.T., Angew. Chem. Int. Ed., 41:3677 (2002); (b) Gan, Z., Reddy, P.T., Quevillon, S., Couve-Bonnaire, S. and Arya, P., Angew. Chem. Int. Ed., 44:1366 (2005); (c) Katritzky, A.R. and Rees, C.W., Comprehensive heterocyclic chemistry Eds, 3, Pergamon. Oxford, 737 (1984).
- Shan, R., Velazquez, C. and Knaus, E. E., J. Med. Chem., 47:254 (2004); (b) Sawada, Y., Kayakiri, H., Abe, Y., Mizutani, T., Inamura, N., Asano, M., Hatori, C., Arsmori, I., Oku, T. and Tanaka, H., J. Med. Chem., 47:2853 (2004).Triggle, D. J. in: Emmet, J. C. (Eds.), Comprehensive Medicinal Chemistry, Vol. II, Pergamon, Oxford (1990).
- (a) Zamponi, G.W., Stotz, S. C., Staples, R. J., Andro, T. M., Nelson, J. K., Hulubei, V., Blumenfeld, A. and Natale, N. R., J. Med. Chem., 46:87 (2003); (b) Visentin, S., Rolando, B., Di Stilo, A., Frutterro, R., Novara, C., Roussel, C.,Vanthuyne, N. and Gasco, A., J. Med. Chem., 47:2688 (2004).
- Eharkar, P. S., Desai, B., Gaveria, H., Varu, B., Loriya, R., Naliapara, Y., Shah, A. and Kulkarn, V. M., J. Med. Chem., 45:4858 (2002).
- Poindexter, G. S., Bruce, M. A., Breitenbucher, J. G., Higgins, M. A., Sit, S. Y., Romine, J. L., Martin, S. W., Ward, S. A., McGovern, R. T., Clarke, W., Russell, J. and Antal-Zimanyi, I., Bioorg. Med. Chem., 12:507 (2004).
- Klusa, V., Drugs Future, 20:135 (1995).
- Bretzel, R. G., Bollen, C. C., Maeser, E. and Federlin, K. F., Drugs Future, 17:465 (1992).
- (a) McCormack, J. G., Westergaard, N., Kristiansen, M. and Brand, C. L., J. Lau. Curr. Pharm. Des., 7:1457 (2001); (b) Ogawa, A. K., Willoughby, C. A., Raynald, B., Ellsworth, K. P., Geissler, W. M., Myer, R. W., Yao, J. and Georgianna, C. K. T., Bioorg. Med. Chem. Lett., 13:3405 (2003).
- (a) Bretzel, R. G., Bollen, C. C., Maester, E., Federlin, K. F., Am. J .Kidney. Dis., 21:54 (1993); (b) Bretzel, R. G., Bollen, C. C., Maester, E. and Federlin, K. F., Drugs Future, 17:465 (1992); (c) Boer, R. and Gekeler, V., Drugs Future, 20:499 (1995).
- (a) Chen, Y. L., Fang, K. C., Sheu, J. Y., Hsu, S. L. and Tzeng, C. C., J. Med. Chem. 44:822 (2001); (b) Roma, G., Braccio, M. D., Grossi, G. and Chia, M., Eur. J. Med. Chem., 35:1021 (2000); (c) Maguire, M. P., Sheets, K. R., McVety, K., Spada, A. P., Zilberstein, A., J. Med. Chem., 37:2129 (1994).
- Hantzsch, A., Justus Liebigs Ann. Chem., 1:215 (1882).
- Ji, S. J., Jiang, Z. Q., Lu, J. and Loh, T. P., Synlett, 831(2004).
- Sridhar, R. and Perumal, P. T., Tetrahedron, 61:2465 (2005).
- Khadikar, B. M., Gaikar, V. G. and Chitnavis, A. A., Tetrahedron Lett., 36:8083 (1995).
- O¨ hberg, L. and Westman, J., Synlett, 1296 (2001).
- Agarwal, A. and Chauhan , P. M. S., Tetrahedron Lett., 46:1345 (2005).
- Kumar, S., Sharma, P., Kapoor , K. K. and Hundal, M. S., Tetrahedron., 64:536 (2008).
- Maheswara, M., Siddaiah, V., Rao, Y. K., Tzeng, Y. and Sridhar, C., J. Mol. Catal. A: Chem., 260:179 (2006).
- Gupta, R., Gupta, R. and Paul, S. L., Synthesis, 2835 (2007).
- Karade, N. N., Budhewar, V. H., Shinde, S. V. and Jadhav, W. N., Lett. Org. Chem., 4:16 (2007).
- Das, B., Ravikanth, B., Ramu, R. and Rao, B. V., Chem. Pharm. Bull., 54:1044 (2006).
- Tewari, N., Dwivedi, N. and Tripathi, R. P., Tetrahedron Lett., 45:9011 (2004).
- Sridhar, R. and Perumal, P. T., Tetrahedron, 61:2465 (2005).
- Debache, A., Boulcina, R., Belfaitah, A., Rhouati, S. and Carboni, B., Synlett, 509 (2008).
- Sabitha. G., Reddy G. S. K. K., Reddy, C. S. and Yadav, J. S., Tetrahedron Lett., 44:4129 (2003).
- Ko, S. and Yao, C. F., Tetrahedron, 62:7293 (2006).
- Reddy, C. S. and Raghu, M., Chin. Chem. Lett., 19:775 (2008).
- Wang, L. M., Sheng, J., Zhang, L., Han, J. W. Fan, Z., Tian, H. and Qian, C. T., Tetrahedron, 61:1539 (2005).
- Donelson, J. L., Gibbs, R. A. and De, S. K., J. Mol. Catal. A:Chem., 256:309 (2006).
- Cherkupally, S. R., Chem. Pharm. Bull., 56:1002 (2008).
- Lee, J. H., Tetrahedron Lett., 46:7329 (2005).
- Kumar, A. and Maurya, R. A., Tetrahedron Lett., 48:3887 (2007).
- Hudlicky, T., Chem. Rev., 96:3 (1996).
- (a) Domling, A. and Ugi, I., Angew. Chem Int. Ed., 39:3168 (2000); (b) Yu, L., Chen, B., and Huang, X., Tetrahedron Lett., 48:925 (2007).
- Corma, A., Current Opinion in Solid State & Materials Science Current Chemistry, Ltd. 2:63 (1997).
- Corma, A. And Garcia, H., Catal. Today, 38:257 (1997).
- Anastas, P. T. and Williamson, T. C., Green Chemistry, Frontiers in Benign Chemical Syntheses and Processes, Oxford University Press, New York, (1998).
- Wender, P. A., Handy, S. L. and Wright, D. L., Chem. Ind., 765 (1997).
- (a) Nasr-Esfahani, M., Karami, B., Montazerozohori, M. and Abdi, K., J. Heterocycl. Chem., 45:1183 (2008); (b) Nasr-Esfahani, M., Montazerozohori, M., Moghadam, M., Mohammadpoor-Baltork, I. and Moradi, S., Phosphorus. Sulfur. Silicon, 185:261 (2010); (c) Nasr-Esfahani, M., Montazerozohori, M. and Gholampour, T., Bull. Korean Chem. Soc., 31:3653 (2010); (d) Nasr-Esfahani, M., Montazerozohori, M. and Mehrizi, S., J. Heterocycl. Chem., 48:249 (2011); (e) Nasr-Esfahani, M., Hoseini, S. J. and Mohammadi, F., Chin. J. Catal., 32:1484 (2011).
- Frederickson, L. D. and Hausen, D. M., Anal. Chem., 35:818 (1963).
- (a) Sapkal, S. B., Shelke, K. F., Shingate, B. B. and Shingare, M. S., Tetrahedron Lett., 50:1754 (2009); (b) Maheswara, M., Siddaiah, V., Lakishmi, G., Damu, V. and Rao, C. V., Arkivoc, 2:201 (2006)06; (c) Mobinikhaledi, A., Foroughifar, N., Bodaghifard, M. A., Moghanian, A., Ebrahimi, S. and Kalhor M., Synth. Commun., 39:1166 (2009).
- Hong, M., Cai, C. and Yi, W. B., J. Fluorine Chem., 131:111 (2010).
- Dabiri, M. and Bashiribod, S., Molecules, 14:1126 (2009).
- Baghbanian, S. M., Khaksar, S., Vahdat, S. M., Farhang, M. and Tajbakhsh, M., Chin. Chem. Lett., 21:563 (2010).

This work is licensed under a Creative Commons Attribution 4.0 International License.









