Synthesis, Cure and Thermal Properties of Bismaleimide Resin and Allylnadic-Imide
I. J. Singh and Hari Om Sharma
Department of Chemistry, C.C.R.(P.G.) College, Muzaffarnagar, India.
Synthesis ofBismaleimidei.e.bis (4-maleimidophenyl) Sulphone (B) and Michael adduct of B with 4,42 - Di amino Diphenyl methane and blended with allylnadicimides i.e. bis (4-allylnadicimidophenyl) methane (S) ,1,6-allylnadicimidohexane(V) , and N-allylnadic-imide(L) was carried out at 280 ºC for 1hr. The cure behavior of the blend resins was measured by DSC.The thermal stability of cured resins was characterized by thermogravimetricanalysis.The thermal stability of the resins improved markedly with the higher content of chain extended bismaleimide (M) with solid allylnadic-imide resin in blends.
KEYWORDS:Bismaleimide; Allylnadic-imide resin; Blends; Thermal properties
Download this article as:| Copy the following to cite this article: Singh I. J, Sharma H. O. Synthesis, Cure and Thermal Properties of Bismaleimide Resin and Allylnadic-Imide. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Singh I. J, Sharma H. O. Synthesis, Cure and Thermal Properties of Bismaleimide Resin and Allylnadic-Imide. Available from: http://www.orientjchem.org/?p=22993 |
Introduction
Addition polyimide are low molecular weight, imide prepolymer send- capped with reactive groups such as acetylenic or olefinic.The olefinic end group in addition polyimides may be cycloaliphatic (i.e. norborene) or aliphatic (i.e. bismaleimides) in nature. Among addition polyimide,bismaleimides (BMIs) ,as a kind of high performance materials have received more and more interest because of their good thermal stability, excellent processability, aerospace structure composities (1), fire resistance and excellent resistance to humidity at elevated temperatures (2-4).
These low molecular weight monomers and oligomers cure thermally to yield a highly cross linked infusible polymer network which is insoluble in all organic solvents. However, due to their highly cross linked structures, bismaleimide resins are extremely brittle and thus are often modified for application (5-9). Cross-link density of maleimide resins has been successfully reduced by chain extension reaction with appropriate nucleophiles (Michael addition reaction) such as diamines (10,11), amino acid hydrazide (12),etc. Stoichiometric mixing of bismaleimide with diamine leads to the formation of linear polyaspartimides which could be cast into a flexible film with poor thermal stability(13).
The main drawback in the used of neat BMI resin is its highly brittle characterstic after curing, caused by its aromatic molecular structure and high cross link density. The problem becomes exacerbated in composite structures. Blending of addition polyimides with several thermoset resins and thermoplastics has been carried out in the past with an aim to improve the processability and phosphorus containing nadimide resin (14) yielded products with improved char yield and better processability.
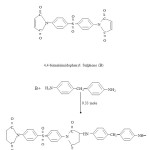
Effect of blending bismaleimide monomer and chain extended bismaleimides with allylnadic-imides on curing characteristic and thermal stability of cured network is now being reported here. Two resin samples B and M having the structure shown in scheme-1, were synthesized for this purpose.
Experimental procedure
Materials
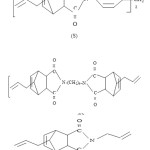
Na2CO3 (BDH), KOH pellets (E.Merck),glacial acetic acid (BDH) methyl ethyl ketone (MEK,BDH), chloroform (E.Merck) and maleic anhydride were used as such. Acetic anhydride (BDH) was distilled before use. The diamines4,4′ diaminodiphenyl methane (Fluka),4,4′ diaminodiphenylsulphone (fluka) were dried bismaleimidophenyl Sulphone (B) under vaccum. Acetone (BDH) was dried over K2CO3 (BDH) and distilled before use. Anhydrous sodium acetate (BDH) was obtained by fusion. Allylnadic-imides bis (4-Allylnadic-imido phenyl) methane (S), 1,6-Allylnadic-imidohexane (V) and N-allylnadic-imide (L) of the structure shown in scheme-2, were procured from Ciba-Geigy.
 |
Scheme 1 Click here to View scheme |
 |
Scheme 2 Click here to View scheme |
Structure of allylnadic-imides (letter in parenthesis indicate the designation of these resins)
Synthesis of Bismaleimide B
In a three nackedflask equipped with a condenser and nitrogen inlet tube, 0.5 mole of 4,4′ diaminodiphenylsulphone was placed and 350 mL of dry acetone was added. The temperature was raised to 60 ºC and solution was stirred by magnetic stirrer. Maleic anhydride (0.10 mole) was then added in several portions and solution was stirred for 2 h at 60 ºC, cyclization of the amic acid intermediate was done by adding fused sodium acetate and acetic anhydride and heating the solution at 60 ºC for another 2h. Bismaleimide was isolated by precipitation using water as a non solvent. The precipitate were repeatedly washed with water and sodium bicarbonate and finally with water. Purification of bismaleimide was done by using silica gel (60-120 mesh) column using chloroform as the solvent. The solution was concentrated on a rotary evaporator and crystals of bismaleimideseprated out on cooling.
Yield = 85.88% m.p.= 247-248ºC
Preparation of chain extended bismaleimide M
Chain extension reaction of B with 4,4′- diaminodiphenyl methane (DADPM) was carried out in acetone/methyl ethyle ketone solution using a molar ratio of 1:0.33. The appropriate quantity of bismaleimide and amine was added to the solvent with continuous stirring and the solution was refluxed for 4 hr till a homogenous solution was obtained. The solvent was removed under reduced pressure using a rotary evaporator and shining powder of bismaleimide-amine adducts were obtained.
Preparation of resin blends
Three blend composition were prepared by dissolving bismaleimide/chain extended bismaleimides (B,M) in acetone and allylnadic-imides (S,V,L) in the ratio of 1:3, 1:1, 3:1.These compositions have been designated by writing the letter designation of bismaleimide/chain extended bismaleimide and allylnadic-imides (S,V,L) and a subscrip indicating the ratio of the two components. For example BS, BS3 and B3S indicate 1:1, 1:3, 3:1 w/w ratio of resin B (bismaleimide) and S (allylnadic-imides) respectively. For homogenous mixing solution technique was used (acetone as solvent). Curing was done at 280ºC for 1hr in a muffle furnace.
Characterization
Structural characterization of various resin samples was done by recording FT-IR spectra in KBr pellets using Perkin- Elmer 580B infrared spectrophotometer 1H-NMR spectra recorded in DMSO-d6 using a Jeol JNM-FX100 FT-NMR spectrophotometer. A DU pont 9900 thermal analyser with 910 DSC Module was used for studying the curing behaviour of resin formulations. A DU pont 1090 thermal analyser with a 951 TG module was used for assessing relative thermal behaviour of resin formulations. Thermogravimetric traces of 10±2 mg size samples were recorded in nitrogen atmosphere (flow rate 50mL/min) at a heating rate of 10ºC/min .
Result and Discussion
In the IR spectra of various maleimideresin sample (Fig. 1) the characterstics carbonyl group was observed at 1780±5 cm-1 and 1720±5cm-1. The other prominentabsorptions arising due to phenyl group 1576±10 cm-1 and 1490±10cm-1), C-N (1370±5 cm-1) and SO2 group (1150±5 cm-1 and 1330±5cm-1). In the chain extended bismaleimide, presence of N-H group was indicated by absorption band at 3380-3400cm-1 (fig.1). Existence of methyl group in the chain extended M resin confirmed from the C-H stretching vibrations which appears at 2950 and 2879cm-1 (Fig 2).
 |
Figure1: FT-IR spectrum of resin B |
 |
Figure 2: FT-IR spectrum of resinM
|
In 1H-NMR spectrum aromatic proton appeared as a multiplet at 7.22-8.08 ppm and olefinic protons at 6.49 ppm. A singlet due to methylene group at 3.34 ppm was observed. These studies confirmed the structure of various samples.
The melting and curing behaviour of these resins was investigated by DSC. A sharp endotherm associated with melting was observed in bismaleimide B at 247ºC. Exothermic transition associated with curing was observed in the temperature range of 248-350ºC. The exothermic peak temperature, Texo, was observed at272ºC (Fig 3). Presence of the strong electron withdrawing group (Sulfone) in B resin may be responsible for the observed increase in the curing temperature. Heat of curing (∆H) was found to be 77.5J/g for B resin. The Texo decreased on chain extension of B with amines and the value of 220ºC was observed in sample M.
 |
Figure 3: (a) TG trace of cured resin B |
In allylnadic-imide resins the curing exotherm was observed in the temperature range of 230-350ºC. Based on the results of the DSC scan, it was decided to carry out the isothermal curing of various resin samples at 280ºC for 1 hr.
Thermogravimetric analysis of cured resin samples was done to evaluate relative thermal stability. Typical TG trace is shown in fig. 3(a) & (b). The initial decomposition temperature (Ti) and temperature of maximum rate of weight loss (Tmax) were noted down from these traces and the results are summarized in theTable-1.Char yield at 800ºC was also determined. The highest char yield of 49% was observed in M sample and lowest value was in allylnadic-imide L (16%). The highest char yield in cured M can be attributed to more cross linking (oxidative coupling of methylene group) in this resin system,which is initiated by benzylic methylene groups leading thereby to formation of condensed ring system.
 |
Figure 3: (b) TG trace of cured resin M |
 |
Figure 4: Plot of char yield (N2 atmosphere, 800 ˚c) Vs weight fraction S in the blends of bismaleimide resin B and M
|
 |
Figure 5: Plot of char yield ( N2 atmosphere, 800 ˚c) Vs weight fraction L in the blends of bismaleimide resin B and M. Click here to View figure |
Blending of bismaleimide B with allylnadic-imides affected the thermal stability and the char yield changed with the composition of the blend. An increase in bismaleimide content resulted in an increase in the char residue in these blends (Table-2). The char yields in the co-cured resin samples were also calculated by rule of mixtures. The observed char yields were higher than the calculated values. A synergistic improvement in the char yield was observed in blends of bismaleimide B and allylnadic-imide resin S (fig 4). However replacement of S by V or L did not show such synergism (fig 5).
Blending of chain extended bismaleimide and allylnadic-imide also affected the thermal behavior of the cured resins (Table-3). The highest char yield 45% was observed in M3S blends. In these resin samples also a synergistic behavior was observed when allylnadic-imide S was used (fig 3). Whereas with resin L no synergism was observed with bismaleimide BS (fig 4). An improvement in char yields was observed when chain extended bismaleimide sample M was used along with V or L in the blends (fig 4).
Co-curing of resin blends may result in a polymer network comprising both constituents. Thermal behavior therefore would be influenced by the structure of network formed. An improvement in the thermal stability on co-curing of allylnadic-imide S with different bismaleimide samples was observed. This synergism may be explained on the basis of co-curing of resins, proposed earlier for a two component high performance resin system based on bismaleimide and 2,2′-diallyl bis phenol-A (14-16). Co-polymerisation of the constituent takes place via ene type linear chain extension reaction followed by Diels-Alder adduct formation.
Table 1: Thermal behavior of cured resin samples
| Resin Formulation
B M S V L |
Ti ºC 468 394 481 470 475 |
Tmax 488 434 497 505 495 |
Yc % 42 49 29 19 16 |
Table 2: Thermal behavior of cured bismaleimide B and allylnadic imide blends
|
Resin Formulation
|
Ti ºC |
Tmax
|
Yc % |
|
BS3 BS B3S BV3 BV B3V BL3 BL B3L |
438 437 467 457 463 468 444 438 452 |
473 469 490 503 493 491 475 473 471 |
38 43 44 26 31 37 25 33 36 |
Table 3: Thermal behavior of cured chain extended bismaleimide and allylnadic-imide blends
|
Resin Formulation
|
TiºC |
Tmax
|
Yc % |
|
MS3 MS M3S MV3 MV M3V ML3 ML M3L |
429 414 402 432 405 426 427 408 399 |
468 457 454 475 449 476 461 446 440 |
41 43 45 25 35 30 31 41 42 |
References
- Stenzenberger H.D., Adv. polymer science, 117,165 (1994).
- Varma I.K. and Gupta V.B. Thermosetting resins-properties in 2nd volume of comprehensive compositic materials, Talreza R. & Manson J.A.E., Ed. Kelly A & Zwebon C., Eds in chief, (Elsevier), 2, (2001).
- Madan R., Srivastava A., Anand R.C. and Varma I.K., Prog. Polym. Sci., 23,621 (1998).
- Lin S.C. and Pearce E.M., High performance thermosets, (Hancer, Munich), 13-61, 187-219, 221-246 (1994).
- Meng J.R., Hu X. and Boey F.Y.C., Lil polymers, 46,2766 (2005).
- HR, Hu X., polymer, 45,9011 (2004).
- Lin Y.L. and Chen Y.J, polymer, 45, 1797 (2004).
- Jeng R.J., Chang C.C., Chen C.P., Chen C.T. and Su W.C., polymer, 44,143 (2003).
- Shilians F., Freddy Y.C. and Abadie M.J.M., Eur. Polym. Journal, 44, 2123-2129 (2008).
- Varma I.K., Gupta A.K. and Sangita, J. Polym. Sci., Polym. Lett Edn., 20,621 (1982).
- Varma I.K., Sangita and Ralli D., J. Polymer News, 12,294 (1987).
- Stenzenberger H.D., US Pat., 421160 – 421161 (1980).
- Crivello J.V., J. Polym. Chem. Ed., 11,313 (1973).
- Mathur A. and Varma I.K., Angew Makromol. Chem., 206,53 (1993).
- Chaudhari M., Galvin T., King J., SAMPE J., 21,17 (1985).
- King J.,Chaudhari M. and Zahir S., 20th national SAMPE Symposium, SAMPE, Covina, Chem. Abstr., 29, 37 (1984).
- Segal L., Stenzenberzer H.D., Herzog M., Romer W., Pierce S. and Canning M., Int SAMPE Techn. Conf., 17,147 (1985).

This work is licensed under a Creative Commons Attribution 4.0 International License.









