Synergistic Effect of Tham- Molybdate And Zn2+ on the Inhibition of Corrosion of Mild Steel in Aqueous Environment
J. Jeyasundari* and Y. Brightson Arul Jacob
Department of Chemistry, NMSSVN College, Madurai - 625 019, India.
The synergistic effect of THAM –Molybdate and Zn2+ on the inhibition of corrosion of mild steel in aqueous environment containing 60 ppm Cl- has been evaluated by the weight loss method. The mechanistic aspects of corrosion inhibition have been studied. The nature of the protective film has been analyzed by using potentiostatic polarization study, impedance spectra, U.V-visible absorption spectra and fluorescence spectra. The formulation consisting of THAM (50ppm) and 50 ppm of Zn2+- SM (250 ppm) has 82% corrosion inhibition efficiency.
KEYWORDS:Synergistic effect; Corrosion inhibition; Carbon steel; AC impedance; FTIR
Download this article as:| Copy the following to cite this article: Jeyasundari J, Jacob Y. B. A. Synergistic Effect of Tham- Molybdate And Zn2+ on the Inhibition of Corrosion of Mild Steel in Aqueous Environment. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Jeyasundari J, Jacob Y. B. A. Synergistic Effect of Tham- Molybdate And Zn2+ on the Inhibition of Corrosion of Mild Steel in Aqueous Environment. Available from: http://www.orientjchem.org/?p=23171 |
Introduction
Molybdate anion has been widely used as corrosion inhibitor since, 1939, because of its efficacy towards both ferrous and non ferrous metal and its very low order of toxicity. Molybdate has been used as inhibitor in combination with nitrite (1-3), alkyl amine phosphate (4-6) and azoles like benzotriizole and tolyltriazole(7). Several studies have been reported on the synergistic effect of Molybdate-Zinc system (8-10). Lizloves (11) have investigated the corrosion inhibition behavior of molybdate in the presence of aggressive chloride and sulfate ions, and reported its efficiency in the presence of oxygen. There have been extensive researches on the improvement the inhibition efficiency of molybdate in actual situation. Farr and Saremi (12) have investigated the effect of immersion time on the corrosion inhibition efficiency of molybdate and the effect of citric acid as a primary film former. Nitrite is an oxidizing agent with corrosion inhibitive properties. It has been used for improving the corrosion inhibition efficiency of molybdate (13).In present study, the synergistic effect of THAM-Zn2+ and Molybdate on the inhibition of corrosion of mild steel in aqueous solution containing 60ppm Cl– has been evaluated by the weight loss method. The mechanism of corrosion inhibition has been studied using potentiostatic polarization study, impedance spectra, and FTIR spectra.
Materials and Methods
Preparation of the mild steel specimens.
Mild steel specimens were chosen from the same sheet of the following composition 0.1 percent C, 0.026 percent S, 0.06 percent P, 0.4 percent Mn and the balance Fe. Mild steel specimen of the dimensions 1.0×4.0x0.2 cm were polished to mirror trichloro ethylene and used for mass loss and surface examinations studies.
Ac impedance measurements
EG and G electrochemical impedance analyzer model 6310 was used to record AC impedance measurements. A Three electrode cell assembly was used. Working electrode, platinum as the counter electrode and saturated calomel electrode as the reference electrode
FTIR Spectra
These spectra were recorded in a Perkin-Elmer 1600 spectrometer. The film was carefully removed, mixed thoroughly with KBr and made in to pellets and the FTIR spectra were recorded.
Potentiostatic polarization study
This study was carried out in a three electrode cell assemble connected to bio analytical system electrochemical analyzer, provided with IR compensation facility.
Results and discussion
Weight loss method
The inhibition efficiencies (IE) and corrosion rates of THAM in controlling corrosion of mild steel immersed in an aqueous solution containing 60 ppm Cl– for a period of three days in the presence and absence of Zn2+ by weight loss methods are given in table 1 and 4. THAM alone has 52 IE. However the formulation consisting of 50 ppm of Zn2+ and 50 ppm of THAM shows 62% IE. It is observed that when SM is added, the inhibition efficiency of THAM increases. The increases in IE is more pronounced at 250 ppm of SM synergistic effect exists between THAM-Zn2+and SM (250pm). Their combination THAM (50 ppm) – Zn2+ (50ppm)-SM (250 ppm) has 82% IE.
Table 1: Corrosion rates of (CR mdd) carbon steel immersed in an aqueous solution containing 60ppm of Cl– and the inhibition efficiencies (IE %) obtained by weight loss method. pH=8.84
|
Cl– ppm |
THAM ppm |
Zn2+ ppm |
CR mdd |
IE % |
|
60 |
0 |
0 |
23 |
– |
|
60 |
0 |
50 |
17.48 |
24 |
|
60 |
50 |
50 |
8.74 |
62 |
|
60 |
100 |
50 |
11.04 |
52 |
|
60 |
150 |
50 |
13.34 |
42 |
|
60 |
200 |
50 |
13.57 |
41 |
|
60 |
250 |
50 |
13.80 |
40 |
Table 2: Corrosion rates (CR mdd) carbon steel immersed in an aqueous solution containing 60ppm if Cl– and the inhibition efficiencies (IE%) obtained by weight loss method.
|
Cl– ppm |
SM ppm |
Zn2+ ppm |
CR mdd |
IE % |
|
60 |
0 |
0 |
23 |
– |
|
60 |
50 |
0 |
19.55 |
15 |
|
60 |
100 |
0 |
15.64 |
32 |
|
60 |
150 |
0 |
13.57 |
41 |
|
60 |
200 |
0 |
11.04 |
52 |
|
60 |
250 |
0 |
9.43 |
59 |
Table 3: Corrosion rates of (CR mdd) carbon steel immersed in an aqueous solution containing 60 ppm of Cl– and the inhibition efficiencies (IE%) obtained by weight loss method .
|
Cl– ppm |
SM ppm |
Zn2+ ppm |
CR mdd |
IE% |
|
60 |
0 |
0 |
23 |
– |
|
60 |
0 |
50 |
17.48 |
24 |
|
60 |
50 |
50 |
14.95 |
35 |
|
60 |
100 |
50 |
12.88 |
44 |
|
60 |
150 |
50 |
11.50 |
50 |
|
60 |
200 |
50 |
7.82 |
66 |
|
60 |
250 | 50 | 5.06 |
78 |
Table 4: Corrosion rates (CR mdd) carbon steel immersed in an aqueous solution containing 60 ppm Cl– and the inhibition efficiencies (IE%) obtained by weight loss method .
|
Cl– ppm |
SM ppm |
Zn2+ ppm |
THAM ppm |
CR mdd |
IE% |
|
60 |
0 |
0 |
0 |
23 |
– |
|
60 |
0 |
50 |
0 |
17.48 |
24 |
|
60 |
0 |
50 |
50 |
8.74 |
62 |
|
60 |
50 |
50 |
50 |
7.59 |
67 |
|
60 |
100 |
50 |
50 |
6.90 |
70 |
|
60 |
150 |
50 |
50 |
5.75 |
75 |
|
60 |
200 |
50 |
50 |
4.6 |
80 |
|
60 |
250 |
50 |
50 |
4.14 |
82 |
Analysis of results of potentiodynamic polarization study
This study has been used to study the formation of protective film on the metal surface. The potentiodynamic polarization curves of carbon steel immersed in an aqueous solution containing 60 ppm of Cl– in the absence and presence of inhibitors are shown in figure 1a.
The corrosion parameters namely corrosion potential (Ecorr) table slopes, bc and ba, linear polarization resistance (LPR) and corrosion parameters (Icorr) are given in table 5. It is observed (figure 1) that when carbon steel immersed in an aqueous solution containing 60 ppm of Cl– the corrosion potential is -578mV vs SCE (saturated calomel electrode). The LPR value is 1.46 x103 ohm cm2.The corrosion current value is 4.293 x10-5 A/cm2) [14].When 50 ppm of THAM , 50 ppm of Zn2+ and 250 ppm of SM are added to the above environment the corrosion potential is shifted to the anodic side (-571 mV vs SCE) figure 1b.
This indicates that the corrosion potential is shifted to the noble side due to the formation of protective film on the metal surface. This is not much change in the value of (221 and 233 mV/decade). But here is slight change in the anodic Tafel slope (415 and 294mV/ decade).This indicates that anodic reaction is controlled predominantly. Hence the THAM – Zn2+-SM system functions as anodic inhibitors. It observed from table 5 that the LPR value increases and the corrosion current value decreases. These observations suggest the formation of a protective film on the metal surface. This prevents the corrosion of metal.
Table 5: Corrosion parameters of carbon steel immersed in an aqueous solution containing 60 ppm of Cl– obtained from potentio dynamic polarization study.
|
System |
Ecorr mV vs SCE |
ba mV/decade |
bc mV/decade |
LPR ohm cm2 |
Icorr A/cm2 |
| Aqueous solution containing 60 ppm Cl– |
-578 |
415 |
221 |
1.46×103 |
4.293×10-5
|
| Aqueous solution containing 60 ppm Cl– + THAM (50ppm) +Zn2+ (50 ppm)+SM (250 ppm) |
-571 |
294 |
233 |
2.270×103 |
2.496×10-5 |
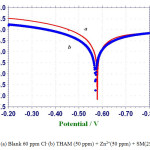 |
Figure 1: (a) Blank 60 ppm Cl– (b) THAM (50 ppm) + Zn2+(50 ppm) + SM(250 ppm). |
Analysis of AC impedance spectra
AC impedance spectra of carbon steel immersed in aqueous solution containing 60 ppm Cl– in the absence and presence of inhibitors are shown in figure 2 (Nyquist Plots) and figure 3 (Bode plots).
The corrosion parameters namely charge transfer resistance (Rt) and double layer capacitance (Cdl) derived from Nyquist plots are given in table 6. The impedance, log (Z/ohm), values derived from bode plots are also given in table 6. It is observed that when carbon steel immersed in an aqueous solution containing 60 ppm Cl– the Rt value is 428 ohm cm2. The Cdl value is 1.19×10-8 F /cm2. The impedance value [log (Z/ohm)] is 2.683 from
When inhibitors (50 ppm of THAM+ 50 ppm of Zn2+ + 250 ppm SM are added the Rt value increases from 428 to 1266 ohm cm2 figure 2b. The Cdl value decreases from 1.19 x 10-8 to 0.40 x10-8 F/cm2. The plot suggests that it is a diffusion controlled process. The impedance value increases from 2.683 to 3.224 can be identifying from figure 2b. This observation suggests that a protective film is formed on the metal surface (15, 16).
Table 6: Corrosion parameters of carbon steel immersed in an aqueous solution containing 60 ppm of Cl– obtained from AC impedance spectra.
|
System |
Rt ohm cm2 |
Cdl F/cm2 |
Impedance [log(Z/ohm)] |
| Aqueous solution containing 60 ppm Cl– |
428 |
1.19×10-8 |
2.683 |
| Aqueous solution containing 60 ppm Cl– + THAM (50 ppm)+Zn2+ (50 ppm + SM (250 ppm) |
1266 |
0.40×10-8 |
3.224 |
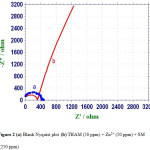 |
Figure 2 (a) Blank Nyquist plot (b) THAM (50 ppm) + Zn2+ (50 ppm) + SM (250 ppm). |
 |
Figure 3a: AC impedance spectra of carbon steel immersed in various test solutions.(Bode Plot) 60 ppm Cl‑ (Blank) |
 |
Figure 3b: AC impedance spectra of carbon steel immersed in various test solutions (Bode Plot) THAM (50 ppm) + Zn2+ (50 ppm) + SM (250 ppm). |
Analysis of FTIR spectrum
The structure of THAM is shown in scheme 1. (Tris buffer)
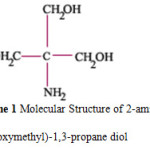 |
Scheme 1: Molecular Structure of 2-amino-2(hydroxymethyl)-1,3-propane diol. |
FTIR spectra have been used to analyze the protective film formed on the meal surface. The FTIR spectrum of the film formed on the film formed on the metal surface after immersion in the solution containing 60 ppm Cl–, 250 ppm of SM, 50 ppm THAM and 50 ppm of Zn2+ is shown in figure 4. The MoO stretching frequency shifted from 829cm-1 to 820cm-1 .the -OH stretching frequency has shifted from 3350 cm-1 to 3403 cm-1. The C-N- stretching frequency has shifted from 1042cm-1 to 1021cm-1. This suggests that SM and THAM coordinated with Fe2+ through their polar groups resulting in the formulation of Fe2+-SM complex and Fe2+ THAM complex. the peak at 1384 cm-1 is due to Zn-O stretching .The –OH stretching frequency appears at 3350 cm-1(17,18).
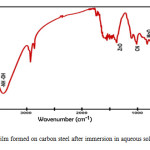 |
Figure 4: Film formed on carbon steel after immersion in aqueous solution containing THAM (50 ppm) +Zn2+(50 ppm) + SM (250 ppm) |
Conclusion
The results of the weight loss study shows that the formulation consisting of 50 ppm THAM and 50 pm of Zn2+ and 250 ppm of SM has 82% IE in controlling corrosion of carbon steel in 60m of Cl– . A synergistic effect exists between Zn2+ and THAM and SM. Polarization study reveals that this formulation functions as anodic inhibitors. AC impedance spectra reveal that a protective film is formed on the metal surface. FTIR spectra reveal that the protective film consists of Fe2+-SM complex and Zn(OH)2. In order to explain these facts the following mechanism of corrosion inhibition is proposed.When the solution containing 60 ppm of Cl– 50 ppm Zn2+ and 50 ppm of THAM and 250 ppm of SM is prepared there is formulation of Zn2+ – THAM, Zn2+-SM complex in solution.When carbon steel is immersed in this solution the Zn2+ – THAM, Zn2+ – SM complex diffuses from the bulk of the solution towards metal surface.On the metal surface Zn2+ – THAM – SM complex is converted in to Fe2+ – THAM, Fe2+ – SM complex on the anodic sites. Zn2+ is released.
Zn2+ – THAM, Zn2+ – SM + Fe2+ –à Fe2+ – THAM, Fe2+ – SM + Zn2+. The released Zn2+ combines with OH– to form Zn(OH)2 on the cathodic sites.
References
- A.L Bayes,US Patent 2147395(1939).
- D.R. Robitaille, Materials Performance, 40, 15(11), (1976).
- F.C.Sessa and R.M.Post, The Betz Indicator, 9, 52(3), (1981).
- R.J.Lipinski and R.F. Weidner, Heating/Piping/Air Conditioning, 103, 50(1), (1978).
- T.C.Breske, Materials Performance, 17, 16(2), (1977).
- D.R.Robitaille and J.G.Bilek, Chem Eng, 79, 83(12), (1976).
- C.O.Neal, Jr.R.N.Burger,Materials Performance ,9,15(2), (1976).
- M.A.Stranick, Proc.4th Intl.Conf,Chemistry & uses of Molybdenum, Amax Inc., Greenwich,CT.August123 (1982).
- D.M.Drazic and L.Z.Vorkapic,Corrosion Science,18,907 (1978).
- M.S.Vukasovich and D.R.Robitaille, J.Less Common Metals, 54,437 (1977).
- E. A. Lizlovs, Corrosion 32, 263 (1976).
- J. P. G. Farr and M. Saremi, Surf. Technol,17, 199 (1982).
- C. M. Mustafa and S. M. S. I. Dulal, British Corros. J. 32, 133 (1997).
- H.Vaidyanadran, H.Hackermann, Corros.Sci, 11,137 (1971).
- Bernd Nowack and Alan T.Stone, Journal of Colloid and Interface Science, 214, 20-30 (1999).
- F. Hanna, G.M. Sherbini, Y. Barakat, Br. Corr. J, 17,131 (1982).
- I.Sekene and V.Hirakawa, Corrosion, 42,206 (1986).
- S.Rajendran,B.V.Apparao,N.Palaniswamy,Proc.8thEurop.Symp.Corros.Inhibitors, Ferrara, Italy,1,465 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









