Spectral and Theoretical Studies of the Stability Constant of Charge Transfer Complexes of a Number of Schiff Bases with Eu(fod)3 in Different Solvents
L. M. N. Saleem, E. A. S. Al-Hyali and F. H. M. Al-Taei
Chemistry Department, College of Education, University of Mosul, Iraq.
The equilibrium constants of charge transfer complexes (CTC) of salicylidene amino pyridines and salicylidene aniline with lanthanide shift reagent Eu(fod)3 in different solvents were determined by applying Benesi- Hildebrand equation. The effect of the polarity of the solvent, electronic factors and steric effect were investigated. Structural and energetic parameters like bond lengths, bond angles, energies of HOMO and LUMO orbitals, η, µ, and ω were obtained by quantum mechanical calculations employing semi empirical model (Austin Method1, AM1) and an abinitio method represented by Hartree Fock (HF) at base set level 6-311G(d,p).
KEYWORDS:Stability Constant; Charge Transfer; Schiff bases
Download this article as:| Copy the following to cite this article: Saleem L. M. N, Al-Hyali E. A. S, Al-Taei F. H. M. Spectral and Theoretical Studies of the Stability Constant of Charge Transfer Complexes of a Number of Schiff Bases with Eu(fod)3 in Different Solvents. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Saleem L. M. N, Al-Hyali E. A. S, Al-Taei F. H. M. Spectral and Theoretical Studies of the Stability Constant of Charge Transfer Complexes of a Number of Schiff Bases with Eu(fod)3 in Different Solvents. Available from: http://www.orientjchem.org/?p=11884 |
Introduction
Lanthanide shift reagent applications are long known to be confined for NMR studies (1,2). Later they have been used to study the UV spectra of Schiff bases. Thus addition of Eu(fod)3 to benzylidene anilines(3) in n-heptanes and acetonitrile resulted in the appearance of two absorption bands in the UV spectra.
In other study Eu(fod)3 used with salicylidene anilines and their substituents, and the aliphatic amines in cyclohexane forming new complexes which were studied by UV spectroscopy. Also Pr(fod)3 was used with benzylidene anilines in cyclohexane(4,5) and in other solvents where K values were calculated, the thermodynamic parameters for these complexes were also estimated. Salicylidene aniline was studied with Pr(fod)3 in mixed solvents(6), it was noticed that, addition of polar solvent to non polar solvent resulted in disappearance of the CTC band. Schiff bases derived from 2-amino pyridine and 2-amino pyrazine were prepared and identified spectroscopically, it was found that, these Schiff bases are present in two tautomers the keto and enol forms, and the enol form is preferred in non polar solvents(7).
In other study new complexes were synthesized from the reaction of Cr(III) with Schiff bases which result from the condensation of 2-amino pyridine with vaniline and salicylaldehyde, the complexes were identified spectroscopically and fond to be tetrahedral(8).
The aim of the present study is to shed light on the effect of the lanthanide shift reagent Eu(fod)3 on Schiff bases containing amino pyridines and salicylaldehyde in different solvents and have an idea theoretically about the most probable positions for coordination and compare them with salicylidene aniline.
Experimental
Chemical materials
Salicylaldehyde, 2-amino pyridine, 2-amino methyl pyridine, 2-amino-4-methyl pyridine, aniline, 2-amino phenol, and lanthanide shift reagent Eu(fod)3 all the above chemicals are purchased from Fluka and they are with high purity except salicylaldehyde which has been distilled at its boiling point.
The solvents used are cyclohexane, CCl4, chlorobenzene, dichloro methane and acetonitrile. All of these solvents were distilled at their boiling points.
Synthesis of Schiff bases
Schiff bases are prepared according to the procedure of El-Bayomi and his coworker(9) by mixing equimolar amounts of salicylaldehyde with primary amines after dissolving it in absolute ethanol then addition of the solution of amine to the aldehyde gradually with stirring, then refluxing the mixture for (2-6 hrs). The mixture was left to cool; the precipitate was filtered, washed with cold ethanol or methanol, then re-crystallized several times to yield a pure product. IR of the compound was measured.
Preparation of solution and formation of the complex
2ml of the suitable solvent was placed in the UV cell (the sample and the blank), then 100 µl of 10-2 M Eu(fod)3 was added to each cell, after that successive additions (20-70 µl)of the donor (Schiff base) was added to the sample cell, an equivalent amount of the solvent was added to the blank cell to maintain equivalent volumes in both cells. UV measurements were carried out using Shimadzu UV-visible spectrophotometer (UV-1659 pc).
Stochiometry of the complexes
The continuous variation method was applied for the determination of stochiometry.
Determination of K value for the complex
The K values are determined by applying the Bensi Hildebrand equation which can be represented as follow:
Theoretical part
The well known Chem. Office (v.11, 2008) program was used for performing this study. Two quantum mechanical methods were used to establish the required values of angles, bond lengths, electronic charges and the steric interactions. One of these method is a semi empirical and represented by the AM1 model and the second one is an abinitio calculation presented as HF (6-311G (d,p)) method.
Results and Discussion
The UV spectra of the Schiff bases studied in this work showed different absorption maxima before and after addition of Eu(fod)3, given in Table (1). From this table, it can be seen that, addition of Eu(fod)3 in different solvents resulted in the appearance of a new band (Figure (1)), which could be attributed to the formation of CT complex.
Table 1: The values of λmax for the Schiff bases, Eu(fod)3 and the complexes formed from them in different solvents
|
Solvent |
S2AP |
S2A3MP |
S2A4MP |
SA |
BOOHA |
r |
|||||
|
b |
co |
b |
co |
b |
co |
b |
co |
b |
co |
||
|
Cyclohexane |
354 |
440 |
356 |
353 |
441 |
355 |
445 |
292 |
|||
|
CCl4 |
354 |
444 |
293 |
||||||||
|
Chlorobenzene |
353 |
441 |
356 |
443 |
352 |
440 |
342 |
437 |
294 |
||
|
Methylenechloride |
348 |
436 |
353 |
441 |
348 |
437 |
339 |
430 |
284 |
||
|
Acetonitrile |
345 |
450 |
292 |
||||||||
S2AP= Salicylidene-2-amino pyridine, S2A3MP= Salicylidene-2-amino-3-methyl pyridine, S2A4MP= Salicylidene-2-amino-4-methyl pyridine, SA= Salicylidene aniline, and BOOHA = Benzylidene –o-hydroxy aniline.
–λmax b= λmax at maximum absorption of Schiff bases, –λmax b= λmax at maximum absorption of Eu(fod)3, –λmax b= λmax at maximum absorption of complex
Methylene chloride Chlorobenzene
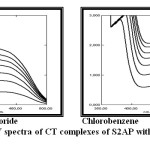 |
Figure 1: UV spectra of CT complexes of S2AP with Eu(fod)3 in two solvents. Click here to View figure |
The stochiometry of this complex was found to be 1:1. Plots were performed between [Ao]/[Abs versus 1/[Do] according to Bensi Hildebrand equation (eq.1).
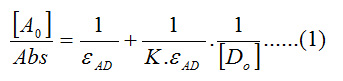
Where [Ao] is the initial concentration of the acceptor (Eu(fod)3), [Do] is the initial concentration of the donor molecule (Schiff base) and εAD is the extinction coefficient of the complex.
Straight lines (Figure (2)) were obtained, where the K values were determined from the slopes of the plots.
 |
Figure 2: Linear relationships obtained from the application of B-H equation on S2AP in various solvents. Click here to View figure |
The values of K for salicylidene 2-amino pyridine in different solvents are given in Table (2). Similarly plots were performed for S2A3MP and SA and BOOHA in two solvents (Chlorobenzene and methylene chloride). The obtained K values for these compounds are tabulated in Table (3).
Table 2: The values of K for the complexes of S2AP with Eu(fod)3 in different solvents.
|
Solvent |
K mol-1 |
|
Cyclohexane |
750 |
|
CCl4 |
428 |
|
Chlorobenzene |
200 |
|
Methylene chloride |
111 |
|
Acetonitril |
40 |
 |
Scheme 1 Click here to View scheme |
Table 3: The values of K for the complexes of Schiff bases with Eu(fod)3 in different solvents
|
Compound |
Solvent |
K mol-1 |
|
S2A3MP |
Chlorobenzene |
50 |
|
Methylene chloride |
20 |
|
|
S2A4MP |
Cyclohexane |
13 |
|
Chlorobenzene |
250 |
|
|
Methylene chloride |
142 |
|
|
SA |
Methylene chloride |
100 |
|
Chlorobenzene |
67 |
|
|
BOOHA |
Chlorobenzene |
7 |
From the above results, it can be seen that, many factors play role in determining the values of K in the studied system. If we look at the values of K for S2AP with Eu(fod)3 in solvents with different polarities, it can be observed that, these values are high in non polar solvent (Table (1)), This could be attributed to the interaction between these polar solvents with the Schiff base because of the presence of the nitrogen atoms in the imine group and/or the pyridine ring in addition to the hydroxyl group which make it difficult for Eu(fod)3 to interact with the Schiff base in the presence of these polar solvents, i.e., the salvation (which is a sort of interaction) causes shielding of the Schiff base and preclude its interaction with the shift reagent, thus lowering the value of K.
Additional observation can draw the attention if we compare the K values of the following Schiff bases: S2AP, S2A3MP, and S2A4MP when interacting with the Eu(fod)3; it can be seen that, these values changes according to the following order
|
Solvent |
S2A3MP |
S2AP |
S2A4MP |
|
Chorobenzene |
50 |
200 |
250 |
|
CH2Cl2 |
20 |
111 |
142 |
This variation can be explained in terms of the electronic effect on the nitrogen of the imine group, thus the presence of a methyl group in the
para position with respect to the imine group in S2A4MP increases the electron density on the nitrogen atom compared to that of the compound with the methyl group in the meta position which makes the first compound more able to interact with the Eu(fod)3 and therefore increases its K value.
On the other hand the methyl group in compound S2A3MP in the ortho position to the nitrogen of the pyridine ring increases the electron density on this ring while in compound S2A4MP the reverse occurs, thus the possibility of interaction between the Schiff base an Eu(fod)3 will be lowered.
The difference in values of K between S2A3MP and S2A4MP could also be attributed to the steric effect which resulted from the presence of the methyl group in a position which is close to the coordination center compared to the second Schiff base S2A4AP causing weakening of the interaction with Eu(fod)3 which result in lowering of K values.
Also we can explain the steric effect if we accept the idea that, the interaction of large molecule like Eu(fod)3 with the Schiff base allow to form eight member ring rather than the six member ring i.e. the interaction take place with the pyridine nitrogen rather than the imino nitrogen. The reasons which make the eight member ring preferred over the six member ring can be explained as follow: if we compare the K values of the complexes obtained from the reaction of Eu(fod)3 with the bases S2AP (K=200) and SA (K=67):
 |
Scheme 2 Click here to View scheme |
The formation of six member rings in both complexes would never explains the great difference of the K values between them so there is a high probability of the formation of a stable eight member ring with the S2AP molecule that releasing some of the steric effect which may be resulted due to spatial congestion raised by a large molecule such as Eu(fod)3 . In addition, if we compare the stability of the two complexes resulted from the reaction of Eu(fod)3 with the Schiff bases SA and BOOHA we can see that the stability of the SA complex (K=67) which form a six member ring is greater than that of BOOHA complex (K=7) which form five member ring.
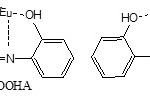 |
Scheme 3 Click here to View scheme |
Further evidences supported these expectations are supplied by theoretical studies.
Theoretical Study
This study is carried out by applying the semi-empirical Austin method1 (AM1) for the estimation of a number of electronic and structural parameters relating to the Schiff bases under consideration. The bases parameters were considered as the most effective and controlling the K values since the other part of the complex (Eu(fod)3) is kept constant.
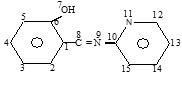
The energies of the HOMO (EHOMO) and LUMO (ELUMO) orbitals, hardness (η), electronic chemical potential (μ) and global electrophilicity (ω) were calculated using equations (2, 3, and 4) respectively and tabulated in Table (4). In addition a number of bond lengths and bond angles belonging to the active part in the structures of the Schiff bases were determined for the same purpose.
Table 4: Values of the electronic and structural parameters of Schiff bases calculated by AM1 method.
|
Comp. |
Solvent |
HOMO |
LUMO |
∆(L – H) |
W |
||
|
S2AP |
Methylene chloride |
-10.043 |
-4.804 |
5.239 |
2.619 |
-7.423 |
10.518 |
|
Chlorobenzene |
-10.053 |
-4.799 |
5.254 |
2.627 |
-7.426 |
10.495 |
|
|
CCl4 |
-10.997 |
-4.715 |
6.282 |
3.141 |
-7.856 |
9.824 |
|
|
Cyclohexane |
-10.167 |
-1.969 |
8.198 |
4.099 |
-6.068 |
4.491 |
|
|
S2A3MP |
Methylene chloride |
-9.995 |
-4.617 |
5.378 |
2.689 |
-7.306 |
9.925 |
|
Chlorobenzene |
-10.057 |
-2.472 |
7.585 |
3.792 |
-6.264 |
5.173 |
|
|
S2A4MP |
Methylene chloride |
-10.969 |
-2.350 |
8.619 |
4.309 |
-6.659 |
5.145 |
|
Chlorobenzene |
-9.001 |
-4.590 |
4.411 |
2.205 |
-6.795 |
10.469 |
|
|
Cyclohexane |
-9.984 |
-4.665 |
5.319 |
2.659 |
-7.324 |
10.086 |
|
|
SA |
Methylene chloride |
-10.125 |
-4.675 |
5.450 |
2.725 |
-7.400 |
10.047 |
|
Chlorobenzene |
-10.210 |
-4.518 |
5.692 |
2.846 |
-7364 |
9.527 |
|
|
BOOHA |
Chlorobenzene |
-9.824 |
-4.064 |
5.760 |
2.880 |
-6.944 |
8.371 |
** Units of energetic values are ev.
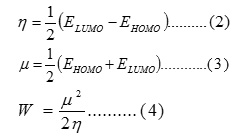
In order to explain the variation noticed in Table (4) and find a connection with the values of K, a simple comparison can be made among the bases S2AP, S2A3MP, and S2A4MP which have similar structures (they differ only by substituting H atom by methyl group) in certain solvent (chlorobenzene). Table (4) shows that, the values of K are reversely proportional to the difference between ELUMO and EHOMO (∆(L – H) or with the values of (η). These variations agree with the principle that says “the less stable molecule is the most active one”.
Looking at the charge present on the nitrogen atom of the pyridine ring (Np), we find that, the existence of ortho methyl relative to this atom in the molecule S2A3MP makes it higher than the analogous atoms in the S2AP and S2A4MP molecules. In the same time, the presence of a methyl group in position meta to the imine nitrogen (Ne) of the S2A3MP does not affect the charge on this atom as a result the charge on Ne become less with respect to the similar in the other molecules.
In contrast the existence of methyl group in position para to the Ne of S2A4MP increases the charge on this atom and does not affect the charge on the Np. Meanwhile, the lower value of (ω) in S2A3MP that makes it behaves as a nucleophile more than the other molecules, becomes greater electrons donating and more liable to form a complex. In fact, the results obtained are in converse with the expected results according to the above discussion. This could be attributed to the steric factor which dominate the interactions of a large molecule (Eu(fod)3) with such Schiff bases and favor the formation of eight member ring.
Such expectation is more clearly noticed when comparing these parameters of the molecules BOOHA, SA and S2AP:
|
Base |
K(mol-1) |
∆(L-H) |
Charge O |
Charge Ne |
μ |
W |
|
|
BOOHA |
7 |
5.760 |
-0.2672 |
-0.1555 |
2.880 |
-6.944 |
8.371 |
|
SA |
100 |
5.692 |
-0.2515 |
-0.1514 |
2.846 |
-7.364 |
9.527 |
|
S2AP |
200 |
5.254 |
-0.2491 |
-0.1356 |
2.627 |
-7.426 |
10.495 |
In this comparison; the principle that, the more stable molecule is less active toward the formation of complex is also being applied. Also we find that, the values of (ω) and the atomic charges (Ne and O) are totally opposing the values of K. This is support the previous assumption in which the electronic parameter is not the dominant factor and the steric effect is controlling the value of K and facilitate the formation of eight member ring complex.
Additional support can come from the values of some structural parameters namely, bond length and bond angels listed in Table (5).
Table 5: Bond lengths and angles of the active parts of the Schiff bases.
|
Base |
K(mol-1) |
C1 – C8 |
C8 – N9 |
N9 – C10 |
|
S2A3MP |
50 |
1.4695 |
1.2878 |
1.4214 |
|
S2AP |
200 |
1.4682 |
1.2917 |
1.4271 |
|
S2A4MP |
250 |
1.4680 |
1.2918 |
1.4210 |
|
|
< C6 C1 C8 |
< C2 C1 C8 |
< N9 C10 C15 |
< N9 C10 N11 |
|
S2A3MP |
120.137 |
120.755 |
119.909 |
117.598 |
|
S2AP |
121.807 |
120.302 |
124.284 |
114.719 |
|
S2A4MP |
121.810 |
120.246 |
124.053 |
115.067 |
Looking carefully at the values in Table (5) we see that, the increase of K values is accompanied by the increase of (< C6 C1 C8) and decrease of (< C2 C1 C8) angle values. In the same time the values of the bond (C1-C8) lengths are decreased and the values of (C8-N9) is increased. On the other side of molecule the values of (< N9 C10 N11) is decreased and being inclined towards the angle (< C6 C1 C8) in the opposite direction which may refer to the position of connection with the reagent (Eu(fod)3) and forming the complex.
References
- K. Binnemans, K. Lodewyckx, T. N. parac- Vogt, R. V. Deun, Bart Goderis, B. Tinat, K. V. Hecke and L. V. Meervelt, “Adducts of Schiff bases with tris(β-diketonato) lanthanide (III) complexes: structure and liquid- crystalline behavior”, Eur. J. Inorg. Chem., 3028- 3033 (2003).
- Sievers, Robert E.,”Lanthanide chelate of a fluorinated ligand”, file//I/4206132. htm., p1-4(1980).
- S. T. Sulaiman, L. M. Saleem and A. S. Authman, “Thermodynamic study of the Interaction of cinnamylidene aniline derivatives with Eu(fod)3 in acetonitrile and n-heptane solvent”, Iraq J. Chem., 24, 49(1998).
- M. G. Al- Dabbagh, “Spectroscope study of charge transfer complexes of salicylidenes and its substituents with Pr(fod)3 in cyclohexane”, M. Sc. Thesis, Mosul University, (2005).
- L. M. Saleem and M. G. Al-dabbagh, “Study of charge transfer complexes of salicylidenes and its substituents with Pr(fod)3 in cyclohexane by U. V. spectroscopy” Raf. Sci., 17, 49, 59-67(2006).
- R. T. Ali, “Spectroscopic study of charge transfer complexes of salicylidene aniline and its derivatives with lanthanide shift reagent Pr (fod)3 in different solvents”, M. Sc. Thesis, Mosul University,(2006).
- A. M. Asir and K. O. Badahdah, “Synthesis of some new anils: part1. reaction of 2-hydroxy benzaldehyde and 2-hydroxy naphtha- aldehyde with 2-aminopyridene and 2-aminopyrazine”, www.mdpi.org/molecules, 12, 1796-1804(2007).
- R. K. Dubey, U. K. Dubey and C. M. Mishra, “Synthesis and physicochemical characterization of some Schiff-bases complexes of chromium (III)”. Ind. J. Chem., 47A, 1208-1212(2008).
- M. A. El-Bayomi, M. El-Asser and Abdel-Halim, “Electronic spectra and structures of Schiff bases. I. benzanils”, J. Am. Chem. Soc., 93, 586(1971).

This work is licensed under a Creative Commons Attribution 4.0 International License.









