Solvent-free Dehydration of Alcohols using LiCl-acidic Alumina
G. Bagheri Marandi¹, A. Pourjavadi²* and H. Hosseinzadeh³
¹Department of Chemistry, Islamic Azad University, Karaj Branch, P. O. Box 31485-313, Karaj, Iran. ²Department of Chemistry, Sharif University of Technology, Azadi Ave., P.O. Box 11365-9516, Tehran, Iran. ³Department of Chemistry, Payame Noor University, P.O. Box 19395-4697, Tehran, Iran.
Conjugated enynes, dienes and simple olefins have been cleanly and selectively synthesized, good-to-excellent yields, by dehydration of corresponding alcohols using focused microwave irradiation on the surface of LiCl-acidic alumina.
KEYWORDS:Conjugated enynes; Dehydration; Microwave; Alumina; Solvent-free
Download this article as:| Copy the following to cite this article: Marandi G. B, Pourjavadi A, Hosseinzadeh H. Solvent-free Dehydration of Alcohols using LiCl-acidic Alumina. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Marandi G. B, Pourjavadi A, Hosseinzadeh H. Solvent-free Dehydration of Alcohols using LiCl-acidic Alumina. Available from: http://www.orientjchem.org/?p=11875 |
introduction
During recent years, the microwave irradiation in conjunction with the solid supported catalysts was used to perform a wide range of organic reactions.1,2 The most advantages for these solvent-free microwave-assisted reactions are the high reaction yields, improvement in reaction selectivity, remarkable practical and handling simplicity, decreasing in reaction rate and without the need for the solvents that are toxic and expensive. In addition, possibility of recovery of both products and supported reagents leading to an efficient and low waste route that achieve the societal, economic and environmental benefits.
Alumina and its derivatives are the most widely employed supports, catalyst and reagents in organic syntheses, where surface of them provides a polar environment and play an important role in this reactions.3, 4 Dehydration of alcohols is one of the reactions in that these supported inorganic reagents has been excessively used .5-7
In this work, we report the synthesis of conjugated enynes and alkenes by dehydration of corresponding alcohols over LiCl-acidic Al2O3 (LiCl-H.Al2O3), a modified derivative of alumina as a supported reagent, under microwave irradiation.
Conjugated enynes are important materials that can be used in various fields such as cycloaddition reactions for construction of aromatic rings 8, synthesis of the natural products9 and polymers.10 Sonogashira et al. for the first time reported the synthesis of conjugated enynes via palladium-catalyzed coupling of terminal alkynes with alkenyl halides.11 After this report, many workers modified the Sonogashira-coupling reaction and also suggested new synthetic approaches for preparation of conjugated enynes, 12-17 because of hardly synthesis of vinyl halides containing various functional groups. The dehydration of α-alkynols has also been used for synthesis of conjugated enynes using various reagents such as Tin (II) chloride in 1,3-dimethylimidazolidin-2-one,18 zeolite catalyst19 and polyphosphoric acid trimethysilyl ester.20
In the previous works, we reported synthesis of conjugated enynes selectively via dehydration of α-arylalkynols using classical heating method21 and also using p-TsCl/natural alumina under classical and microwave heating methods.22 In this paper, microwave-assisted dehydration of several α-arylalkynols, and a number of other alcohols was performed by using the LiCl-H.Al2O3 solid supported reagent. Representative results are shown in Table 1. α-Arylalkynols, 1A-8A, were synthesized using Sonogashira reaction,11,23 are selectively converted to the corresponding conjugated enynes, 1P-8P, in good yields, in comparison to α-β-unsaturated carbonyl compounds that can be obtained via Meyer-Schuster rearrangement24 (Scheme 1).
Table 1. Dehydration of alcohols using microwave irradiation on the surface of LiCl-acidic alumina.
 |
Table 1: Dehydration of alcohols using microwave irradiation on the surface of LiCl-acidic alumina. Click here to View figure |
The physical and/or spectroscopic data of arylalkynols, 1A-8A, was reported in the previous work22 and for other alcohols corresponded exactly to those cited in literature. bDetermined by GC.
 |
Scheme 1: Click here to View scheme |
The other alcohols, 9A-16A were also converted to the corresponding alkenes at the same method, in good to excellent yields.It is obvious that alkenols, in acidic media, were converted to conjugated dienes. Therefore, the alkenol 14A, in LiCl-H.Al2O3 media, was selectively converted to the corresponding conjugated diene 14P, via rearrangement of the resulted carbocation (Scheme 2).
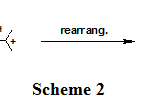 |
Scheme 2: Click here to View scheme |
 
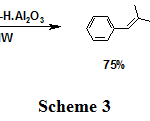
Also, conjugated alkene 13P is the major product of the dehydration of homobenzylic alcohol 13A (Scheme 3).
|
|
Scheme 3: |
 
In our procedure, the alkanol 15A was converted to the more substituted alkene 15P (Scheme 4).
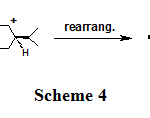 |
Scheme 4: |
 
In conclusion, herein we reported a simple, coefficient and fast method for the preparation of conjugated enynes, dienes and simple olefinic compounds via dehydration of corresponding alcohols using LiCl-H.Al2O3 under MW irradiation.
Experimental
The acidic Al2O3 used Aluminium oxide 90 active acidic (Merck-1078). Column-chromatographic separations were performed on silica gel 60 (Merck-9385). Thin-layer chromatography (TLC) was performed on silica gel 60 (Merck-7748) plates and visualisation was effected with short wavelength UV light (254 nm). Melting points were obtained on a Buchi B-540 melting point apparatus. MW experiments were performed in a Westinghouse 1400 W (900 W output) domenstic MW oven. The arylalkynols were prepared using the Sonogashira reaction and the other alcohols were analytical grade.
General procedure for the microwave-assisted synthesis of conjugated enynes and alkenes:
The certain amounts of LiCl and acidic alumina (indicated in Table 1) were well mixed and then 1 mmol of alcohols was added into the mixture and the mixture was grind using a pestle and mortar. The mixture was poured into a 200 mL Erlenmeyer flask attached to a short column (12×2cm) and was irradiated by microwave with level 10 (high power-900W) for certain time according to Table 1. Then the mixture was cooled to room temperature and the product was extracted with ethyl acetate. After this, the solvent was removed in vacuo to afford a crude product. Finally, the purified product was obtained by column chromatography using hexane as eluent.
References
- L. Perreux and A. Loupy, Tetrahedron, 2001, 57, 9199.
- P. Lindstrom, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225.
- D. Villemin, A.B. Alloum and F. Thibault-Starzyk, Synth. Commun., 1998, 22, 1359
- H. Benhaliliba, A. Derdour, J.P. Bazureau, F. Texier-Boullet and J. Hamelin, Tetrahedron Lett., 1998, 39, 541
- B.F. Plummer, Z.Y. Al-Saigh and M. Arfan, J. Org. Chem., 1984, 49, 2069.
- M. Jayami, N. Pant, S. Anathan, K. Narayanan and C.N. Pilai, Tetrahedron Lett., 1986, 42, 4325.
- I. Hodgetts, S.J. Noyce and R.C. Starr, Tetrahedron Lett., 1984, 25, 5435.
- (a) S. Saito, T. Kawasaki, N. Tsuboya and Y. Yamamoto, J. Org. Chem., 2001, 66, 796. (b) B.R. Kaafarani and D.C. Neckers, Tetrahedron Lett., 2001, 42, 4099. (c) S. Saito, N. Tsuboya, Y. Chounan, T. Nogami and Y. Tamamoto, Tetrahedron Lett., 1999, 40, 7529. (d) H. Kuroda, E. Hanaki and M. Kawakami, Tetrahedron Lett., 1999, 40, 3753. (e) V. Gevorgyan, Y. Tamamoto, J. Organomet. Chem., 1999, 576, 232. (f) S. Saito, Y. Yamamoto and O. Hmori, Org. Lett., 2000, 2 (24), 3853.
- (a) K. Mori, The Total Synthesis of Natural Products, J.A.P. Simon, Ed., John Wiley, New York, 1992, vol. 9. (b) K.C. Nicolaou and W.M. Dai, Angeew. Chem., 1991, 103, 1453. (c) M.E. Maier, Synlett., 1995, 13. (d) J.W. Grissom, G.U. Gunawardena, D. Klingberg and D. Huang, Tetrahedron, 1996, 52, 6453. (e) J.J. Matasi, J. Yan and J.W. Herndon, Inorganica Chimica Acta, 1996, 296, 273. (f) H. Hubner, C. Haubmann, W. Utz and P. Gmeeiner, J. Med. Chem., 2000, 43 (4), 756.
- (a) B. Ochiai and I.T. TomitaEndo, Polymer, 2001, 42, 8581. (b) H. Muller, S.K. Schumidt and M. Hagedorn, Macromol. Rapid Commun., 2000, 21, 449. (c) B. Ochiai, I. Tomita and T. Endo, Macromolecules, 1999, 32, 238. (d) B. Ochiai, I. Tomita, T. Endo, J. Polym. Sci. Part A: Polym. Chem., 2001, 39, 1016. (e) V. Gevorgyan, K. Tando, N. Uchiyama and Y. Yamamoto, J. Org. Chem., 1998, 63, 7022.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 4467.
- S. Thorand and N. Krause, J. Org. Chem., 1998, 63, 8551.
- M. Yoshida, S. Yoshikawa, T. Fukuhara, N. Yoneda and S. Hara, Tetrahedron, 2001, 57, 7143.
- C.H. Oh, H.R. Sung, S.H. Jung and Y.M. Lim, Tetrahedron Lett., 2001, 42, 5493.
- U. Halbes, P. Bertus and P. Pale, Tetrahedron Lett., 2001, 42, 8641.
- V. Cadierno, M.P. Gamasa and J. Gimeno, J. Organomet. Chem., 2001, 621, 39.
- G. Zeni and J.V. Comasseto, Tetrahedron, 1999, 40, 4619.
- Y. Masuyama, J.U. Takahara, K. Hashimito and Y. Kurusa, J. Chem. Soc., Chem. Commun., 1993, 1219.
- G. Sartori, A. Pastorio, R. Maggi and F. Bigi, Tetrahedron, 1996, 52, 8287.
- M. Yoshimatsu, H. Yamada, H. Shimizu, T. Kataoka, J. Chem. Soc., Chem. Commun., 1994, 2107.
- A. Pourjavadi and G. Bagheri Marandi, J. Chem. Res. (S), 2002, 378.
- A. Pourjavadi and G. Bagheri Marandi, J. Chem. Res. (S), 2002, 552.
- (a) K. Sonogashira, Comprehensive Organic Chemistry, I. Fleming and B.M. Trost, Eds., Pergamon, Oxford, 1991, vol. 3, p. 521. (b) S. Takahashi, Y. Kuroyama, K. Sonogashira and N. Hagihara, Synthesis, 1980, 627.
- K.H. Meyer and K. Schuster, Chem. Ber., 1922, 55, 819.
- S. Safe, Can. J. Chem., 1970, 48, 1171.
- R.H. Mitchell, J. Chem. Soc., Chem. Commun., 1974, 990.
- R.H. Dewolfe, J. Am. Chem. Soc., 1957, 79, 4795.
- B.L. Burt, Tetrahedron Lett., 1977, 3063.
- E. Degney, Bull. Soc. Chim. Fr., 1972, 4770.
- P.A. Krapcho and G. E. Jahngen, J. Org. Chem., 1974, 39 (9), 1322.
- L. Boroweicki, Bull. Soc. Chim. Fr., 1967, 2364.

This work is licensed under a Creative Commons Attribution 4.0 International License.