Ring Opening Epoxides Into Halohydrins with Elemental Iodine and Bromine in the Presence Of Nano Catalyst Zro2
Javad Azizian1*, Abolghasem Shameli2, Ebrahim Balali3, Mohammad Mehdei Ghanbari4 and Shahab Zomorodbakhsh5
1Department of Chemistry, Science and Research Branch, Islamic Azad University, P.O. Box 19395-1775, Tehran, Iran. 2Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidyeh ,Iran 3Department of Chemistry, Pharmaceutical Sciences branch, Islamic Azad University, Tehran, Iran. 4Department of Chemistry, Sarvestan Branch, Islamic Azad University, Sarvestan, Iran. 5Department of Chemistry Mahshahr Branch, Islamic Azad University, Mahshahr, Iran.
There is a continued interest in the regioselective ring opening of oxiranes to the corresponding vicinal halohydrins. Although a variety of new and mild procedures to effect this transformation have been reported, most of them have some limitations.The ring opening of epoxides with elemental bromine and nano catalyst ZrO2affords vicinal bromo alcohols in high yields. This new procedure occurs regioselectively under neutral and mild conditions in various aprotic solvents even when sensitive functional groups are present.
KEYWORDS:Ring opening; epoxides; regioselective; bromine; nano catalyst; alcohols
Download this article as:| Copy the following to cite this article: Azizian J, Shameli A, Balali E, Ghanbari M. M, Zomorodbakhsh S. Ring Opening Epoxides Into Halohydrins with Elemental Iodine and Bromine in the Presence Of Nano Catalyst Zro2. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Azizian J, Shameli A, Balali E, Ghanbari M. M, Zomorodbakhsh S. Ring Opening Epoxides Into Halohydrins with Elemental Iodine and Bromine in the Presence Of Nano Catalyst Zro2. Available from: http://www.orientjchem.org/?p=22985 |
Introduction
There is a continued interest in the regioselective ring opening of oxiranes to the corresponding vicinal halohydrins. Although a variety of new and mild procedures to effect this transformation have been reported, most of them have some limitations.[1]The reaction is typically performed with hydrogen halides, but the harsh reaction conditions and the low observed regioselectivity in the opening of unsymmetrical epoxides have prompted a search for more selective and milder procedures. Recently, it has been found that epoxides can be converted into halohydrins by means of elemental halogen,[2] but this method has limitations such as low yield, long reaction times, low regioselectivity and formation of acetonide byproducts in addition to the expected iodo adduct. Furthermore, iodination does not occur in aprotic solvents other than acetone.
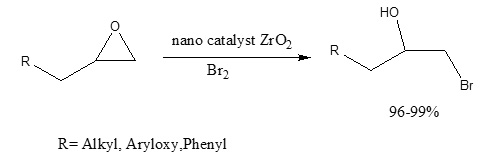
In conjunction with ongoing work in our laboratory on the synthesis and formation of complex heterocyclic compounds containing donor nitrogen atoms, with neutral molecules such as iodine,[3-5] we found out that ZrO2 with frame nano efficiently catalyzed the addition of elemental bromine to epoxides under mild reaction conditions with high regioselectivity (Scheme 1).
Scheme 1: synthesis bromohydrin by ZrO
Exprimental
NMR spectra were recorded by a Bruker Avance 300 MHz pectrometer locked on deuterium from solvent. Chemical shifts (d [ppm]) were calculated from chemical shift of deuterium lock and were not calibrated. FTIR spectra were measured on Perkin Elmer 2000 spectrometer in KBr pellets (1/200).
Epoxide (1 mmol) in CH2Cl2 (5 mL) was added to a stirred ZrO2 catalyst (0.15 mmol) in at room temperature. Next, a solution of elemental Bromine (1 mmol) in CH2Cl2 (5 mL) was added portion-wise (15 min) to the above mixture. The progress of the reaction was monitored by TLC. After complete disappearance of the starting material, the reaction mixture was washed with 10% aqueous Na2S2O3 (2×10 mL) and water (2×10 mL). The aqueous layer was extracted with CH2Cl2 (2×10 mL).The combined organic layer was dried over anhydrous MgSO4 and evaporated to give crude alcohol–catalyst.
Result
In this study, we wish to report the results of the reactions of some epoxides with elemental bromine and Iodine in the presence of a sub-stoichiometric amount of ZrO2 (Scheme 1, Table 1).
Table 1: ring opening 3-phenoxy-1,2-epoxypropane.
| ENTRY | SOLVENT | (%mol) catalyst | Time( h) | Isolate yield (%) |
|
1 |
– |
10 |
6 |
50 |
|
2 |
THF |
10 |
8 |
50 |
|
3 |
MeOH |
10 |
8 |
30 |
|
4 |
EtOH |
10 |
8 |
25 |
|
5 |
CCl4 |
10 |
8 |
30 |
|
6 |
CH3CN |
10 |
5 |
50 |
|
7 |
H2O |
10 |
24 |
trace |
|
8 |
CH2Cl2 |
10 |
10min |
80 |
|
9 |
CH2Cl2 |
15 |
5min |
98 |
|
10 |
CH2Cl2 |
20 |
5min |
98 |
The crude products were purified on a column of silica gel. The solvent was evaporated and pure halohydrin was obtained. The halohydrins obtained throughout this procedure were identified by comparison, where possible, with authentic samples prepared in accordance with literature procedures.
Bromo -2-boutanol (99%)
1HNMR (CDCl3, 300MHz) δ 0.95 (t, 3H, J=7.2Hz), 1.55-1.7 (m, 2H), 3.05-3.25(m, 1H), 3.3-3.5 (m, 1H), 3.75-3.8(m, 1H).; MS(EI) M/Z 200( M+)
Bromo Cyclohexanol (97%)
1HNMR (CDCl3, 300MHz) δ 1.2-1.6(m, 4H), 1.8-1.9 (m, 1H), 2.0-2.2(m, 2H), 2.4-2.5(m, 1H), 3.5-3.65(m, 1H), 3.95-4.05(m,1H);MS(EI) M/Z 226( M+); IR(KBr) 3425, 2960 cm-1
Bromo -1-(4-Cholrophenyl)ethanol (96%)
1HNMR (CDCl3, 300MHz) δ 2.45(br, 1H), 3.45-3.5 (m, 2H), 4.80-4.88(m, 1H), 7.2-7.4(m, 4H);MS(EI) M/Z 282( M+); IR(KBr) 3460, 2960 cm-1
Bromo -1-phenyl ethanol (98%)
1HNMR (CDCl3, 300MHz) δ 2.50(br, 1H), 3.39-3.5 (m, 2H), 4.75(m, 1H), 7.25-7.4(m, 5H);MS(EI) M/Z 248( M+); IR(KBr) 3398 , 2960 cm-1
Bromo -3-(4-methoxyphenyl)2-propanol (98%)
pale yellow liquid, 1HNMR (CDCl3, 300MHz) δ 2.05(br, 1H), 2.85(d, 2H, J=6.2, 9.2Hz), 3.25 (dd, 1H, J=4.8,9.2 Hz), 3.35 (dd, 1H, J=3.8,9.2Hz), 3.6-3.75(m, 1H), 3.80(S, 3H),6.85(d, 2H,J=8.2Hz), 7.15(d, 2H, J=8.2Hz); 13CNMR (CDCl3,50Hz) δ 14.67, 41.66, 55.10, 71.62, 113.92, 128.98, 130.12, 158.25;MS (EI) M/Z 292 (M+); IR(KBr) 3560, 3050, 2960 cm-1
Bromo -3-(4-acetylphenoxy)2-propanol (99%)
yellow Solid, mp 68-700 C, 1HNMR (CDCl3, 300MHz) δ 2.55(S, 3H), 3.30-350 (m,2H), 3.90-4.05 (m, 3H), 6.90(d, 2H,J=7.8Hz), 7.9(d, 2H, J=7.8Hz);13CNMR (CDCl3,50Hz) δ 8.84, 26.21, 69.12, 70.15, 114.66, 130.64, 162.75, 196.96; MS (EI) M/Z 320 (M+); IR(KBr) 3460, 3020, 2970, 1710 cm-1
Bromo -3-(4-Cholorophenoxy)2-propanol (97%)
1HNMR (CDCl3, 300MHz) δ 2.40 (br, 1H), 3.35-3.40 (m,2H), 3.45-3.50 (m, 1H), 3.95-4.0 (m, 1H), 4.05-4.10(m, 2H), 6.85(d, 2H, J=8.2 Hz), 7.15(d, 2H, J=8.2 Hz); MS (EI) M/Z 312 (M+); IR(KBr) 3515 cm-1
Bromo-3-phenoxy-2-propanol(98%):
1HNMR (CDCl3, 300MHz) δ 2.40(br, 1H), 3.30-3.55 (m, 2H), 3.8-4.1(m, 3H), 6.75-7.0(m, 3H) 7.15-7.35(m,2H);MS(EI) M/Z 278(M+); IR (KBr) 3500 , 2985 cm-1
Discussion
In conclusion, this new method appears to be highly competitive with the other methods reported in the literature. The reaction occurs in neutral and mild conditions on the acid-sensitive substrates and vicinal halohydrins were obtained in high yields and region-selectivity. In addition, in comparison with our previous methods, ZrO2 is cheaper, less step need for preparation, and overall yield is higher.
Acknowledgment
This study financially supported by Islamic Azad University Omidyeh branch. We thanks’ Mr. Mohammad Reza Asgharei for valuable helps.
Reference
- Fiesers’ Reagents for Organic Synthesis, Smith, J. G., Fieser, M., Eds.; New York, (1990); looking for: halohydrins and derivatives. (b) Bonini, C.; Righi, G. Synthesis, 225-260 (1994). (c) Larock, R. C. Comprehensive Organic Transformations. VCH: New York,; pp 508–509 (1989).
- Konaklieva, M. I.; Dahi, M. L.; Turos, E. Tetrahedron Lett., 33, 7093-7096 (1992).
- (a) Sharghi, H.; Massah, A. R.; Eshghi, H.; Niknam, K. J. Org. Chem., 63, 1455-1461 (1998). (b) Sharghi, H.; Niknam, K.; Pooyan, M. Tetrahedron, 57, 6057-6064 (2001). (c) Gangali, M. R.; Eshghi, H.; Sharghi, H.; Shamsipur, M. J. Electroanal. Chem., 405, 177-182 (1996). (d) Sharghi, H.; Massah, A. R.; Abedi, M. Talanta, 49, 531-535 (1999).
- Sharghi, H.; Nasseri, M. A.; Niknam, K. J. Org. Chem., 66, 7287-7282 (2001).
- Niknam, K.; Nasehi T. Tetrahedron 58 10259–10261 (2002).
- Zhou, H.; Steinhilber, D.; Schlaad, H; Sisson, A.L; Haag, H.Reactive & Functional Polymers, , 71 ,356–361 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









