Reactions of 2-Thioxoper Hydro-1, 3-Thiazin-4 Ones with Oxovanadium (Iv), Chromium (Iii), Manganese (Ii), Iron (Ii) And Iron (Iii)
B. P. Singh, K. K. Singh1 and J.P. Singh2
1Department of Chemistry, T.D. Post Graduate College, Jaunpur, India. 2Department of Chemistry S.G.R. Post Graduate College Jaunpur, India.
In this paper, the reaction of 2-thioxoperhydro – 1, 3 – thiazin – 4 ones; with VO(IV), Cr(III), Mn(II), Fe(II) and Fe(III) ions lead to the formation of M(TP)X (H2O)Y[M=Mn(II), Cr(III), Fe(III); x=1 or 2; y=2 or 3], VO(TP)2 and [Fe(TPH)Cl2(PPh3)2]. These complexes have been characterized by analytical, magnetic, thermogravimetric and spectral (IR, UV and Visible) studies and their tentative structures are proposed.
KEYWORDS:Spectroscopic methods; Metal complexes; Fungicides; Pesticides
Download this article as:| Copy the following to cite this article: Singh B. P, Singh K. K, Singh J. P. Reactions of 2-Thioxoper Hydro-1, 3-Thiazin-4 Ones with Oxovanadium (Iv), Chromium (Iii), Manganese (Ii), Iron (Ii) And Iron (Iii). Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Singh B. P, Singh K. K, Singh J. P. Reactions of 2-Thioxoper Hydro-1, 3-Thiazin-4 Ones with Oxovanadium (Iv), Chromium (Iii), Manganese (Ii), Iron (Ii) And Iron (Iii). Available from: http://www.orientjchem.org/?p=23163 |
Introduction
It is recognished that the properties of the metal complexes of phasphorus doner ligands1,3 are markedly affected by the electronic and steric effects of the substituents on Phasphorus. However, investigations on the electronic and steric effects in metal complexes of arsenic donar ligands have been lacking. Recently reactions of [Ru (h5-C5H5) (PPh3)2 Cl] with source aromatic thioamides (R1CSNHCOR2) have been reported4. We report here the synthesis and characterisation of the products of [Ru(h5-C5H5)(PPh3)2X] (X=Cl, Br, I), with TPH & [Ru(n5-C5H5) (MPh3)2] (M = As, Sb) and [Rh(CO)2 X] and [Ru(CO)2 X] (X=Cl, Br, I), with TPH respectively.
Experimental
All the chemicals used were either of AR or chemically pure grade 2 – Thioxoperhydro-1, 3-thiazine-4 ones, (TPH) was prepared by the literature method given elswhere5 [Ru(h5-C5H5) (PPh3)2X] (X=Cl, Br, I),6 [Ru(h5-C5H5) (MPh3)2 Cl]7,8 (M = As, Sb), [Ru(CO)2 Cl2]9 and [Rh(CO) X]2 10,11 were prepared by literature methods.
Preparation of metal Complexes
[(h5-C5H5) RuCl (PPh3) (TPH)]
A solution of [(h5-C5H5) RuCl (PPh3)2] (0.182g, 0.25 mmol) and ligand TPH (0.52g ~ 0.35 mmol) in 40 ml MeOH was stirred for 2hrs under a dry N2 atmosphere. The resultant solution was kept in refrigerator for 2 hrs where by a yellowish brown crystalline complexe precipitated. It was centrifuged, washed with MeOH, ether and dried in vacuo.
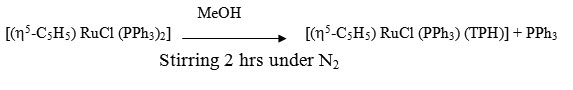
[(h5-C5H5) RuBr (PPh3) (TPH)]
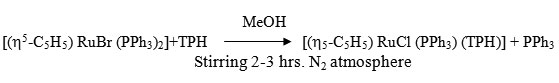
To a 20ml MeOH suspension of [h5-C5H5 RuBr (PPh3)2] (0.19 ~ 0.25 mmol) was added MeOH solution (20ml of ligand (0.05 ~ 0.35 mmol) with constant stirring under dry N2. The stirring was continued for 2-3 hrs. whereby the colour of solution changes from orange to orange brown. It was filtered and filtrate was kept in refrigerator for 2 hrs, an orange brown needle like crystal separated out. It was centrifuged, washed several times with MeOH ether and dried in vacuo.
[(h5-C5H5) RuI (PPh3) (TPH)]
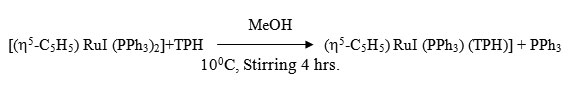
To a 20 ml (CH2Cl2) solution of [(h5-C5H5) RuI (PPh3)2] (0.20g ~ 0.25 mmol) was added drop wise 20 ml MeOH solution of ligand (0.50g ~ 0.35 mmol) with constant stirring at 100C under a dry N2 atmosphere. The stirring was continued for 4 hrs whereby a reddish violet crystalline complex separated. It was centrifuged, washed with methanol, dichloromethane, ether and dried in vacuo.
[(h5-C5H5) RuCl (AsPh3) (TPH)]
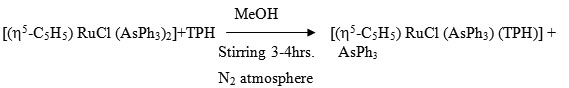
A solution of [(h5-C5H5) RuCl (AsPh3)2] (0.105g ~ 0.25 mmol) and ligand (0.5g ~ 0.35 mmol) in 40 ml was stirred for 2-3 hrs under a dry N2 atmosphere. The resulting solution was evaporated to near dryness the residue extracted by CH2Cl2 and the complex was precipited by using petroleum ether (60-800). It was centrifuged and purified by recrystallization from CH2Cl2 – Petroleum ether (60-800). It was again centrifuged and washed with petroleum ether and dried is vacuo.
[(h5-C5H5) RuCl (SbPh3) (TPH)]
To a continuously stirred 20 ml MeOH solution of [(h5-C5H5) RuCl (SbPh3)2] (0.100 ~ 0.19 mmol) was added dropwise a 20 ml (MeOH) solution of ligand (0.05g ~ 0.35 mmol) at room temperature under dry N2 atmosphere. The stirring was continued for 2-3 hrs where by the colour of reaction changes to reddish brown. It was filtered and the filtrate was kept in refrigerator for 4 hrs where by a reddish brown needle like crystals of the complex separated. It was centrifuged, washed with MeOH, ether and dried in vacuo.
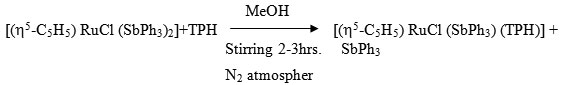
[Ru(CO)2 Cl2 (TPH)]
A MeOH solution (50ml) containing [Ru(CO)2 Cl2] (0.114g ~ 0.5 mmol) and ligand (0.75g ~ 0.5 mmol) was refluxed for 5-6 hrs. The resulting solution was concentrated almost to dryness under reduced presure, The residue was dissolved in minimum quantity of CH2Cl2 followed by an addition of excess (50ml) petroleum ether with constant stirring. On standing for 1-2 hrs, the precipitate settled. It was centrifuged, washed several times with petroleum ether (60-800) and dried in vacuo.
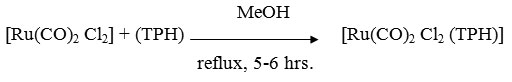
[Rh(CO)2 Cl (TPH)]
A MeOH soluation (50ml) containing [Rh(CO)2 Cl]2 (0.18g ~ 0.5 mmol) and ligand (0.15g ~ 1 mmol) was stirred for 4 hrs at 100C where by the colour of solution changes from red to brown. It was centrifuged and centrifugate was reduced to 20 ml at low pressure and kept in refrigerator for 2 hrs where by a crystaline reddish brown compound was formed. It was centrifuged and washed with methanol, ether and dried in vacuo.
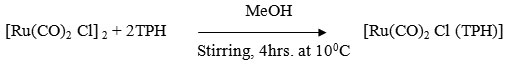
[Rh(CO)2 Br (TPH)]
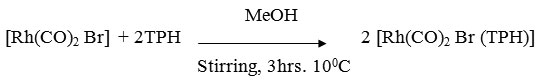
A MeOH solution (50ml) containing [Rh(CO)2 Br]2 (0.23 g ~ 0.5 mmol) and ligand (0.15g ~ mmol) was stirred for 3 hrs at 100C whereby the colour of reaction mixture changes from dark brown to yellowish brown. It was centrifuged and the centrifugate was reduced to 10 ml at low pressure. On adding 50 ml petroleum ether (60-800) a yellowish brown precipitate of complex was formed. It was centrifuged and washed with MeOH, ether and dried in vacuo.
Analyses
A weighed amount of complex was taken in a pyrex beaker. It was decomposed by digesting it with aquaregia for some time until the residue gave the transparent solution. The solution was used for the estimation of sulphur. Halogens were estimated by standard procedures given elsewhere12 as Agx(X=Cl, Br, I). P, As, and Sb were estimated first by decomposing samples with Na2O2, Sugar and NaNO3 in a Parr Bomb, extracted the melt with water and heated it to remove SO3 fumes. It was dilluted with water and filtered. The feltrate was used to estimate P, As and Sb, by the standard procedures. Rhodium was estimated by the standard procedure13 C, H, N analyses were performed by the Microanalytical section of B.H.U., Varanasi, Magnetic susceptibilities of the complexes were determined with the help of Gouy balance at room temperature. IR spectra of the complexes were scanned on Perkin-Elmer spectrophotometer model 580. Melting points of the complexes were observed on Fisher-John melting points apparatus and reported without further correction. Conductivity of the complexes were checked by Elco Model apparatus.
Results and Discussion
Analytical data are consistant with stoichiometry proposed for the complexes (Table-1). The compounds are non-conductors in dichloromethane, generally air stable and soluble in most of the organic solvents and are diamagnetic. In order to propose a probable structures of the complexes according to proposed formulations, the spectral (ir and uv – visible) studies have been carried out on this complexes whose results are discussed under the following heads.
IR spectral studies [(h5-C5H5) RuX (PPh3) (TPH)] (X=Cl, Br, I) and [(h5-C5H5) RuCl (MPh3) (TPH)] (M=As, Sb)
The donor ability of this class of ligand can perhaps be best understood in terms of resonance structure given below.
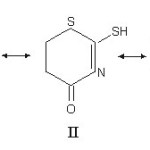 |
Scheme 1 Click here to View scheme |
Ordinarily, only structure I is considered, but II and III may predominate under certain situations. In the case of coordination of the ligand through its carbonyl oxygen atom, n(C=O) should shift to lower wave number and thioamide band I [d (NH + n(CN) should shift to higher wave number. Whereas if coordination is through nitrogen atom, the thioamide band I14, 15 will shift to lower wave number. Thioamide band II [n(C=N)+ n (C=S) + d(C-H)] and III [n (C – N) + n (C – S)]11 do not shift systematically after complexation, have they could not be used for deciding a bonding site. Based on above criterian a bonding mode has been arrived at (Table 2). In all the complexes is n (C=O) of the ligand goes to lower wave numbers after complexation, hence coordination through oxygen is likely to occur. Coordination through ring nitrogen or sulphur is unlikely as they are weakly basic. The p bonding through C=S, C=O and C=N is almost ruled out on the analogy with the bonding of similar type of lignads. The X-ray crystal diffraction of [Cu(PPh3)2– (LH)Cl] shows that coordination occurs through thione sulphur.16,17
A new strong band at around 2350 cm-1 appears which is assigned to n (O-H). This shows that the coordinated ligand is in thiol form. This is further confirm by the disappearance of n (NH), n (C=S) and thioamide band IV. The smaller value of n (S-H) in the complexes may be due to strong intermolecular hydrogen bonding. In addition a new band at 600 cm-1 appears in all the complexes and is assigned to n (C-S). Similar ligands are reported to be predominantly in thioketoforms is solid or solutions. Howerver, enethiolization of thioamides may markedly be enhanced in Presence of metal ions.
All the characteristic bands of PPh3,19AsPh3,20 SbPh3,21,22 and h5-C5H5 (820-850 cm-1)23 were present in the IR shpectra of complexes. The new bands in the region 350-480 cm-1 may be attributed to n (Ru-Cl)+ n (Ru-O) in Ru (h5-C5H5) Cl (MPh3) (TPH) (M=P, As, Sb) where as in [(Ru(h5-C5H5)X(PPh3) (TPH)] the new bands around 260-350 cm-1 are assigned to n (Ru-X) + n (Ru-O)22 (X=Br, I).
[Rh(CO)2 Cl2 (TPH)]
The band due to n (C=O) shifts to higher wave numbers on complexation, excluding the possibility of carbonyl oxygen as donar. The n(C=S) band and the thioamide band IV (mainly due to n (C=S)) shift to lower wave numbers with reduced intersity. This support the bonding through thiocarbonyl sulphur. The thioamide band I (δ(NH) + n (CN)) in complexes shift to lower wave numbers indicating metal nitrogen bond formation. Thioamide band II (n (C=N) + δ (NH) + δ (CH)) and thioamide band III (n (C = S) + n (C – N)), as expected also shift to lower wave numbers. The ligand is probably nitrogen and sulphur donor is these complexes. An intense band at 2040 cm-1 is assigned to n (CºO). This also indicates the presence of two carbonyl ligand in the trans position.25
[Rh(CO)2 X (TPH)]
(i) Ligand band at 3160 cm–1 n (NH) did not change its position (±5cm-1) in the spectra of complexes. This could probably be explained on the assumption that during the formation of complexes, the NH group of the ligand is not participating in the bond formation. The bands due to ~ (NH) at 645 and 680 cm-1 of the ligand were observed in the spectra of complexes without any shift (±5cm-1).
(ii) The band due to n (CO)26 of the ligand at 1710 cm-1 shift to higher wave number by 20 cm-1. This indicates the non involvement of carbonyl oxygen in the bond formation with the metal ion.
(iii) The n (C=S) of the ligand at 1125 cm-1 and at 750 cm-1 (thioamide band IV) shift to lower frequencies (20-25 cm-1).
(iv) The band at 1500 cm-1 in the ligand is assigned to thioamide band I (n (C=N) + δ (C – H). In most of the complexes this band shift to higher wave numbers (Table-2).
(v) The ligand band at 1345 cm-1 assigned to thioamide band II (n (C=S) + n (C – N) + δ (C – N), shifts to lower frequencies (10 – 12 cm-1) in the spectra of complexes.
(vi) The thioamide band III (mainly due to (n (C=S) and n (C – N)) at 1000 cm-1 undergoes downward shift (10 cm-1) an complexation.
(vii) The appearance of an intense band at 1960 cm-1 [Rh(CO)2 Cl (TPH)] and 1940 cm-1 in [Rh(CO)2 (Br) (TPH)] indicates the presence of carbonyl groups in trans position in both the complexes.25
(viii) Two new bands at 300-350 cm-1 in chloro complex may be assigned to coupled vibration of n (Rh-Cl) and n (Rh-S). In bromo complex the bands at 260-320 cm-1 may be similarly assigned to n (Rh-Br) + n (Rh-S) vibrations.24
Magnetic and uv-visible spectra of the complexes.
[(h5-C5H5) Rux (PPh3) (TPH)] (X=Cl, Br, I) and [(h5-C5H5) RuCl (MPh3) (TPH)] and [Rh(CO)2 Cl2 (TPH)]
All the complexes are diamagnetic in solid state. This points towards an octehedral strong field environment of ligands around metal Ru(II) ions (d6 system) and square planer geometry around Rh(I) ions (d8 system) causing a low spin configuration. Table 3 lists the l max of the electronic spectra of the ligand and complexes obtained at room temperature is dichloromethane along with their assignments.
In visible region a rather broad band of medium intensity centering around 450 nm was exhibited by all the complexes. As it is generally believed that d-d or MLCT transition in Ru(II) octahedral complexes occur under a broad envelop at around 450 nm–. The Presence of a similar band in present complexes suggested an octahedral geometry of ligand around Ru(II) ion. The 450 band seems to be ligand dependent, it may be assigned to MLCT.28, 29 The probability of this band being due purely to d-d transition is rather low, because of the availability of low lying empty p* orbitals of the ligand. The strong bands in all the complexes at 340-330 nm may be assigned to intraligand bands.30
The conductivity at room temperature ranges from 8.3 mhos to 56.8 mhos for all the complexes (Table-1) indicating their non ionic nature31 in dichloromethane since no trend of conductivity with composition is evident it is probable that measured conductivites are strongly influenced by impurities. A distorted octahedral geometry is suggested for all the complexes.
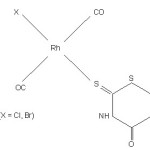 |
Scheme 2 Click here to View scheme |
(X = Cl, Br, I) (M = As, Sb)
In uv-visible spectrum of Rh(I) (ground state 1A2g) complex should exhibit three spin allowed and three spin forbidden bands.32 Besides these the spectrum should show three spin forbidden singlet triplet transitions from 1A2g ® 3A2g, 3B1g and 3Eg respectively. But only two extremely weak bands are present at 540 and 605 nm. These bands are assigned to singlet to triplet transitions 1A2g ® 3A2g , 3B1g in square planar Rh(I) complexes. The third band around 450 nm is masked by the presence of intense charge transfer band.
The condictivity of these complexes in dichloromethane at room temper was found to be 26.8 mohos for [Rh(CO)2Cl(TPH)] and 18.4 mohos for [Rh(CO)2Br(TPH)] indicating their non ionic nature in dichloromethane. Thus, on the basis of analytical, conductivity and magnetic spectral (ir and uv-visible) studies, a square planar geometry is suggested for these complexes.
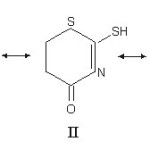 |
Scheme 3 Click here to View scheme |
Table 1 : Analytical data, Colour, Melting points, Yield, Conductivity And Magnetic properties of complexes
|
Compound |
Colour |
Melting points (0C) |
Yield % |
Conductivity |
Magnetic (B.M.) |
Found Calcd (%) |
||||||
|
C |
H |
N |
S |
Cl/Br/I |
P/As/Sb |
Metal |
||||||
| [(h5-C5H5) RuCl (PPh3) (TPH)] | Yellowish brown |
130 |
38 |
50.8 |
D |
52.95 (53.07) |
4.22 (4.12) |
2.19 (2.29) |
10.59 (10.49) |
5.70 (5.80) |
4.99 (5.07) |
16.40 (16.54) |
| [(h5-C5H5) RuBr (PPh3) (TPH)] | Orange brown |
105 |
60 |
48.4 |
D |
49.35 (49.47) |
3.96 (3.84) |
2.03 (2.13) |
9.88 (9.78) |
12.8 (12.19) |
4.60 (4.72) |
15.30 (15.42) |
| [(h5-C5H5) RuI (PPh3) (TPH)] | Reddish violet |
90 |
85 |
8.2 |
D |
46.06 (46.16) |
3.69 (3.58) |
1.88 (1.99) |
9.24 (9.13) |
17.99 (18.07) |
4.21 (4.41) |
14.30 (14.39) |
| [(h5-C5H5) RuCl (PPh3) (TPH)] | Violet |
115 |
42 |
45.6 |
D |
49.43 (49.54) |
3.19 (3.08) |
2.04 (2.14) |
9.90 (9.80) |
5.31 (5.41) |
11.30 (11.45) |
15.34 (15.44) |
| [(h5-C5H5) RuCl (SbPh3) (TPH)] | Grey |
105 |
35 |
24.5 |
D |
46.10 (46.20) |
2.92 (2.87) |
1.87 (1.99) |
9.26 (9.14) |
4.96 (5.05) |
17.23 (17.35) |
14.30 (14.40) |
| [Rh(CO)2 Cl2 (TPH)] | Red brown |
200 |
66 |
30 |
D |
19.10 (19.21) |
1.46 (1.34) |
3.63 (3.73) |
17.20 (17.10) |
18.80 (18.90) |
– |
26.86 (26.95) |
| [Rh(CO)2 Cl (TPH)] | Yellowish brown |
200d |
45 |
26.8 |
D |
21.00 (21.10) |
1.58 (1.47) |
4.01 (4.10) |
18.88 (18.78) |
10.28 (10.38) |
– |
30.04 (30.14) |
| [Rh(CO)2 Br (TPH)] | Dark brown |
140 |
20 |
18.4 |
D |
18.57 (18.67) |
1.40 (1.30) |
3.53 (3.63) |
16.75 (16.62) |
20.60 (20.71) |
– |
26.57 (26.67) |
Table 2 : IR Spectral Bands of Ligands and Complexes (Cm-1)
|
Compound |
n(NH) |
n (C=O) |
n (C=O) |
n (C=S) |
Thioamide bands |
||||
|
I |
II |
III |
IV |
||||||
| C4H5NOS2 |
3160m |
1710s |
– |
1125s |
1540s |
1345s |
1000m |
750s |
|
| [(h5-C5H5) RuCl (PPh3) (TPH)] |
– |
190s |
– |
1135s |
1555s |
1350s |
1005s |
– |
|
| [(h5-C5H5) RuBr (PPh3) (TPH)] |
– |
190s |
– |
1130s |
1560s |
1355s |
1015m |
– |
|
| [(h5-C5H5) RuI (PPh3) (TPH)] |
– |
1690s |
– |
1140s |
1550s |
1340sd |
1010m |
– |
|
| [(h5-C5H5) RuCl (PPh3) (TPH)] |
– |
1690s |
– |
1130s |
1560s |
1340s |
1020m |
– |
|
| [(h5-C5H5) RuCl (SbPh3) (TPH)] |
– |
1695s |
– |
1145s |
1565s |
1350s |
1015m |
– |
|
| [Rh(CO)2 Cl2 (TPH)] |
3125m |
1735s |
2040s |
1120s |
1520s |
1320s |
980m |
740s |
|
| [Rh(CO)2 Cl (TPH)] |
3120m |
1735s |
2020s |
1105s |
1525s |
1325s |
990m |
730s |
|
| [Rh(CO)2 Br (TPH)] |
3100m |
1730s |
2005 |
1110s |
1520s |
1325s |
975m |
725s |
|
Table 3 : Electronic Spectra of the Ligands and Complexes in CH2Cl2
|
Compound |
lmax(nm) |
Assignments |
| C4H5NOS2 |
360s 450s |
p®p* h®p* |
| [(h5-C5H5) RuCl (PPh3) (TPH)] |
355s 460s |
IL CT |
| [(h5-C5H5) RuBr (PPh3) (TPH)] |
350s 450s |
IL CT |
| [(h5-C5H5) RuI (PPh3) (TPH)] |
340s 450s |
IL CT |
| [(h5-C5H5) RuCl (PPh3) (TPH)] |
350s 465s |
IL CT |
| [(h5-C5H5) RuCl (SbPh3) (TPH)] |
350s 465s |
IL CT |
| [Rh(CO)2 Cl2 (TPH)] |
340 450 |
– |
| [Rh(CO)2 Cl (TPH)] |
335 440 540 605 |
IL CT 1A2g®3T2g 1A2g®3B1g |
| [Rh(CO)2 Br (TPH)] |
340 450 540 605 |
IL CT 1A2g®3T2g 1A2g®3B1g |
References
- McAuliffe, C.A., and Levasan, W., Phosphine Arsine and Stibine Compounds of the Transition Elements. Elsevier. Amsterdam (1979)
- McAuliffe (Ed.), C.A., Transition metal Complexes of Phosphine, Arsine Antimony ligands. Wiley, New York (1973)
- Tolman, C.A., Chem. Rev. 313, 77 (1977).
- Gupta, H.K., Chauhan, Veena., and Dikshit, S.K., Inorg. Chim. Acta, 175, 128, (1987).
- Takeshima. T., Fukada. N., Okhi. E. and Muvaoka. M., J. Chem. Res (S); 212 (1979).
- Wilczewski, T., Bochenska, M., and Biernat, J.F., J. Organomet. Chem, 87, 215, (1981)
- Bruce, M.I., Humphrey, M.G., Swincer, A.G., and Wallis, R.C., Aust. J. Chem. 1747, 37, (1984).
- Mohan Rao, K., Mishra, L., and Agarwala, U.C., Ind. J. chem.., 755, 264 (1987).
- Colton, R., and Farthing, R.H., Aust. J. Chem., 1283, 20 (1967).
- Gallay, J., J. Organoment. Chem., 179, 38 (1972).
- Brown, C., J. Organomet. Chem., 93, 192 (1980).
- Vogel, A.T., “A Text-Book of Quantitative Inorganic Chemistry” Longmems, Green and Co. London (1968).
- Beamish F.E. “The Analytical Chemistry of Noble Metals”, Vol. 24, 1st Edn., Pergamou Press, Oxford (1966)
- Rao, C.N.R., Venkataraghvan, R., and Kasturi, T., Can. J. chem., 42, 36, (1967).
- Fabretti, A.C. Ferrari, M., Franchim, G.C., Preti, C., Tasri, L., and Tosi, G., Trans. Met Chem., 279, 7 (1982)
- Singh, B., Rukhaiyar, M.M.P., and Sinha, R.J., J. Inorg Nucl. Chem., 29, 39 (1977).
- Pandey, K.K., Mathias, N., Sheldrick, G.M., and Saheb, R., Naturforsch, Z., 586, 39b (1984).
- Akbar Ali, M., Ghausul Hossain, S.M., Majumder, S.M.M.H., Nazim Usddin, M., and tarafder, M.T.H., Palyhedron, 1653, 6, (1987).
- Sheldon, J.C., and Tyreejr, S.Y., J. Am. Chem. Soc., 2120, 80 (1958).
- Shobatake, K., Postamus, C., Ferraro, J.r., and Nakamoto, K., Apple. Spectrose, 12, 23, (1969).
- Maier, L., Prog. Inorg. Chem., 27, 5 (1963).
- Nakamoto, K., “Infrared and Raman Spectra of Inorganic and Coordination Compounds” John-Willey Publication, 3rd Edn., PP 33, (1977).
- Ashok, R.F.N., Gupta, M., Arulsamy, K.S., and Agarwala, U.C., Inorg. Chim. Acta, 161, 98, (1985).
- Adam, D.M., “Metal Ligand and Related Vibrations”, St. Martins Press new Yark, PP 284, 316, (1968).
- Cotton, F.A., and Wilkinson, G., Advanced Inorgchemistry” John Wiley and Sons., New York, Vth Edn. P 1035 (1988).
- Raja, D.S., Paramaguru, G., Bhuvanesh, N.S.P., Reibenspies, J.H., Renganathan, R., and Natrajan, K., Daton Trans., 4548, 40 (2011).
- Patil, S.A., Naik, V.H., Kulkarni, A.D., Badami, P.S., Spectrochim Acta, 347, 75A (2010).
- Johsnon, C.R., and Shepered, R.E., Inorg. Chem.. 2439, 22, (1983) and references therein.
- Tfouni, E., Doro, F.G., Gomes, A.J., Da Shilva, R.S., Metzker, G., Benni, P.G.Z., and Franco, D.W., Coord. Chem. Rev., 355, 254 (2010).
- Kalaivani, P., Prabhakaran, R., Dallemer, F., Poornima, P., Vaishnavi, E., Ramachundran E., Vijaya Padma, V., Renganathan, R., and Natrajan, K., Metllomics., 101, 4 (2012).
- Lakshmi, S., Int. J. Chemtech. Res. 464, 4 (2012).
- Lever, A.B.P., Inorg. Chem., 763, 4, (1965).

This work is licensed under a Creative Commons Attribution 4.0 International License.









