Preparation and Biological Evaluation of Novel Pyrimidines from Novel Chalcones
M.V. Jyothi1* and P. Venkatesh2
1Department of Pharmaceutical Chemistry, Raghavendra Institute of Pharmaceutical Education and Research, K.R.Palli cross, Anantapur - 515 721, India.
2Department of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Balanagar, Hyderabad - 500 037, India.Pyrimidines are the parent substances of a large group of heterocyclic compounds and play a vital role in many biological processes, as found in nucleic acids, several vitamins, coenzymes, purines and possess therapeutic activities like antimicrobial, anti-inflammatory, anticancer, antiviral, antitubercular and antimalarial activities. In the present study an attempt is made to synthesize pyrimidines from novel chalcones which provide an easy route of synthesis and chalcones themselves possess antimicrobial activity. All these compounds were characterized by means of their IR, 1H NMR, 13C NMR, and mass spectral data. These compounds were evaluated for antimicrobial activity by cup plate method.
KEYWORDS:Pyrimidines; Chalcones; Synthesis; Antimicrobial activity; Cup plate method
Download this article as:| Copy the following to cite this article: Jyothi M. V, Venkatesh P. Preparation and Biological Evaluation of Novel Pyrimidines from Novel Chalcones. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Jyothi M. V, Venkatesh P. Preparation and Biological Evaluation of Novel Pyrimidines from Novel Chalcones. Available from: http://www.orientjchem.org/?p=23153 |
Introduction
Chalcones and pyrimidines were reported to possess various biological activities. In the present communication we report the synthesis of novel chalcones1-11 following claisen-schmidt condensation using 3-acetylpyridine with either aromatic or heteroaromatic aldehydes (2a-f) in the presence of alkali. The resulting chalcones (3a-f) after purification and characterization by physical and spectral methods have been successfully converted into novel substituted pyrimidines12-19 (4a-f) by reaction with guanidine hydrochloride. The structures of the various synthesized compounds were assigned on the basis of elemental analyses, IR, 1H NMR, 13C NMR, and mass spectral data. These compounds were screened for their antimicrobial activity20-22 and the results were reported.
Materials and Methods
Melting points were determined on a capillary melting point apparatus and are uncorrected. 1H NMR and 13C NMR spectra were recorded in the indicated solvent on Bruker AMX 400 MHz spectrophotometer using TMS as an internal standard. Infrared spectra were recorded in KBr on Perkin-Elmer BXF1 spectrophotometer. Microanalyses were performed on carlo Ebra 1108 element analyzer and were within the ± 0.5% of the theoretical values. Column chromatography was performed on silica gel (Merck, 100-200 mesh).
General procedure for the synthesis of novel chalcones
Equimolar quantity (0.001mol) of 3-acetylpyridine and respective aldehydes were mixed and dissolved in minimum amount of alcohol. To this, 40 % aqueous potassium hydroxide solution (15 ml) was added slowly and mixed occasionally for 24 hrs, at room temperature. Completion of the reaction was identified by TLC using Silica gel-G. After completion of the reaction, the reaction mixture was poured into crushed ice, if necessary acidified with dil.HCl. The solid separated was filtered and dried. It was purified by column chromatography on silica gel (100-200 #, Merck), using ethylacetate and hexane mixture (1:1) as mobile phase.
Reaction
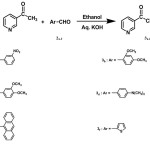 |
scheme 2 Click here to View scheme |
1-(3′- pyridyl)-3-(3”-nitrophenyl)-2-propen-1-one (3a):
Yield 87%; mp 178 oC; Relative molecular mass 254; IR (KBr) 1690 (C=O), 1610 (HC = CH), 1590 (C =N); 1H-NMR 7.50 (1H, d, J=17 Hz , -CO-CH=), 7.86 (1H, d, J=17 Hz, =CH-Ar), 7.65-9.20 (8H, Ar-H).Anal.calcd for C10 H10N2O3: C, 64.56; H, 3.93; N, 11.02. Found: C, 64.57; H, 3.95; N, 11.04.
1-(3′- pyridyl)-3-(2”, 4”-dimethoxyphenyl)-2-propen-1-one (3b):
Yield 86%; mp 156 oC; Relative molecular mass 269; IR (KBr) 1690 (C =O), 1618 (HC =CH), 1596 (C =N); 1H-NMR 3.90 (6H, s, 2 x OCH3), 7.50 (1H, d, J=17 Hz, – CO- CH =), 8.07 (1H, d, J=17 Hz, = CH- Ar), 6.50-9.20 (8H, Ar- H). Anal.calcd for C16 H15NO3: C, 71.37; H, 5.57; N, 5.20. Found: C, 71.39; H, 5.55; N, 5.19.
1-(3′-pyridyl)-3-(3”, 4”-dimethoxyphenyl)-2-propen-1-one (3c):
Yield 75%; mp 138 oC; Relative molecular mass 269; IR (KBr) 1684 (C=O), 1610 (CH=CH), 1590 (C=N), 1210 (C-O-C); 1H-NMR 3.90 (6H, s, 2 x OCH3), 7.01 (1H, d, J=17 Hz , -CO-CH=), 7.38 (1H, d, J=17 Hz, =CH- Ar), 6.85-8.70 (7H, Ar-H) Anal.calcd for C16H15NO3: C, 71.37; H, 5.57; N, 6.28. Found: C, 71.38; H, 5.59; N, 6.26.
1-(3′-pyridyl)-3-(4”-N, N-dimethylaminophenyl)-2-propen-1-one (3d):
Yield 95%; mp 144 oC; Relative molecular mass 252; IR (KBr) 1696 (C=O), 1620 (CH=CH), 1586 (C=N), 1180 (-N-(CH3)2); 1H-NMR 3.05 (6H, s, N Me2), 6.69 (1H, d, J=17 Hz , -CO-CH=), 7.10 (1H, d, J=17 Hz , =CH – Ar), 7.25 – 8.73 (8H, Ar-H). Anal.calcd for C16 H16N2O: C, 76.19; H, 6.34; N, 11.11. Found: C, 76.17; H, 6.32; N, 11.10.
1-(3′-pyridyl)-3-(9”-anthracenyl)-2-propen-1-one (3e):
Yield 95%; mp 78 oC; Relative molecular mass 309; IR (KBr) 1695 (C =O), 1610 (HC =CH), 1592 (C = N), 1528 (C=C); 1H-NMR 7.46 (1H, d, J=17 Hz , =CH-Ar), 7.24 (1H, d, J=17 Hz, -CO-CH=), 7.2 – 8.94 (13H, Ar-H). Anal.calcd for C22 H15NO: C, 90.10; H, 5.11; N, 4.77. Found: C, 90.09; H, 5.13; N, 4.75.
1-(3′-pyridyl)-3-(2”-thienyl)-2-propen-1-one (3f):
Yield 96%; mp 162 oC; Relative molecular mass 215; IR (KBr) 1696 (C=O), 1620 (CH=CH), 1590 (C=N), 650 (C-S); 1H-NMR 7.07 (1H, d, J=17 Hz, -CO-CH= ), 7.39 (1H, d, =CH -Ar), 7.20-8.73 (7H, Ar-H). Anal.calcd for C12H9NOS: C, 66.97; H, 4.18; N, 6.51. Found: C, 64.99; H, 4.16; N, 6.50.
General procedure for the synthesis of pyrimidines :
A mixture of chalcones (obtained by the above method) of 3-acetylpyridine (0.001 mol) and guanidine hydrochloride (0.001 mol) in absolute ethanol (10 ml) were refluxed on a water bath for 6 hours. The solvent was completely evaporated and the residue was poured into ice cold water, the precipitated solid was collected by filtration and crystallized from a suitable solvent to give the desired substituted pyrimidine.
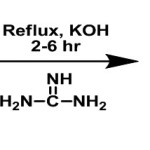 |
scheme 2 Click here to View scheme |
2-amino-4-(3′- pyridyl)-6-(3″-nitrophenyl) pyrimidine (4a):
Yield 72%; mp 265-269 0C; Relative molecular mass 293; IR (KBr) 3342 (NH2), 1642 (C=N), 1586 (C=C), 1358 (C-N); 1H-NMR 7.20 (1H, s, C-5-H), 5.52 (2H, s, C-2- NH2), 7.40-8.70 (8H, Ar-H). Anal.calcd for C15H11N5O2: C, 61.43; H, 3.75; N, 23.89. Found: C, 61.45; H, 3.79; N, 23.91.
2-amino-4-(3′-pyridyl)-6-(2”, 4″-dimethoxyphenyl) pyrimidine (4b):
Yield 65%; mp 238-242 0C; Relative molecular mass 308; IR (KBr) 3316 (NH2), 1680 (C=N), 1570 (C=C), 1340 (C-N), 1210 (C-O-C); 1H-NMR 7.36 (1H, s, C-5-H), 5.52 (2H, s, C-2-NH2), 7.26-8.60 (7H, Ar-H). Anal.calcd for C17H16N4O2: C, 65.59; H, 5.14; N, 18.00. Found: C, 65.60; H, 5.13; N, 18.01.
2-amino-4-(3′- pyridyl)-6-(3″, 4”-dimethoxyphenyl) pyrimidine (4c):
Yield 75%; mp 285-289 0C; Relative molecular mass 311; IR (KBr) 3340 (NH2), 1632 (C=N), 1579 (C=C), 1356 (C-N), 1208 (C-O-C); 1H-NMR 7.30 (1H, s, C-5-H), 5.58 (2H, s, C-2-NH2), 3.95 (6H, 2 x OCH3), 6.90 – 8.69 (7H, Ar-H). Anal.calcd for C17H16N4O2: C, 65.59; H, 5.14; N, 18.00. Found: C, 65.57; H, 5.12; N, 18.02.
2-amino-4-(3′-pyridyl)-6-(4″-dimethylaminophenyl) pyrimidine (4d):
Yield 72%; mp 265-269 0C; Relative molecular mass 266; IR (KBr) 3342 (NH2), 1642 (C=N), 1586 (C=C), 1358 (C-N), 1108 (-N-(CH3)2); 1H -NMR 7.30 (1H, s, C-5-H), 5.30 (2H, s, C-2-NH2), 3.10 (6H, -N-(CH3)2) -6.80– 9.10 (8H, Ar-H). Anal.calcd for C17H17N5: C, 70.10; H, 5.84; N, 24.05. Found: C, 70.06; H, 5.82; N, 24.02.
2-amino-4-(3′-pyridyl)-6-(9″-anthracenyl) pyrimidine (4e):
Yield 75%; mp 295-299 0C; Relative molecular mass 348; IR (KBr) 3340 (NH2),1640 (C=N), 1580 (C=C), 1358 (C-N); 1H-NMR 7.30 (1H, s, C-5-H), 5.60 (2H, s, C-2-NH2), 7.10-8.75 (13H, Ar-H). Anal.calcd for C23H16N4: C, 79.31; H, 4.59; N, 16.09. Found: C, 79.28; H, 4.56; N, 16.10.
2-amino-4-(3′- pyridyl)-6-(2″-thienyl) pyrimidine (4f):
Yield 62%; mp 208-212 0C; Relative molecular mass 254; IR (KBr) 3308 (NH2), 1632 (C=N), 1579 (C=C), 1358 (C-N), 1H-NMR 7.20 (1H, s, C-5-H), 5.30 (2H, s, C-2-NH2), 7.10 – 8.80 (7H, Ar-H). Anal.calcd for C13H10SN4: C, 61.41; H, 3.93; N, 22.04. Found: C, 61.38; H, 3.90; N, 22.01.
Antimicrobial activity
The antibacterial activity of synthesized chalcones and their pyrimidine derivatives was conducted against three Gram-positive bacteria viz., Bacillus pumilis, Bacillus subtilis and Staphylococcus aureus and two Gram-negative bacteria viz., Escherichia coli, Proteus vulgaris by using cup plate method. Preparation of nutrient broth, subculture, agar medium and peptone water was done as per standard procedure. Each test compound (5 mg) was dissolved in dimethylsulfoxide (5 ml) to give a concentration of 1000 mg/ml. All the compounds and the standard were tested at 50 mg (0.05 ml) and 100 mg (0.1 ml) dose levels and DMSO used as a control. Ampicillin as standard drug was also prepared at a concentration of 1000 mg/ml in sterilized distilled water.
All the compounds which were screened for antibacterial activity, also screened for their antifungal activity. The fungi employed for screening were Aspergillus niger, Rhizopus oryzae and Candida albicans. Fluconazole was employed as standard to compare the results. The test organisms were sub-cultured using potato-dextrose-agar (PDA) medium.
Each test compound (5mg) was dissolved in dimethylsulfoxide (5ml) to give a concentration of 1000 mg/ml. Fluconazole solution was also prepared at a concentration of 1000 mg/ml in sterilized distilled water. All the compounds and the standard were tested at 50 mg ( 0.05 ml) and 100 mg (0.1 ml) dose levels and DMSO used as a control.
Results
ANTIBACTERIAL ACTIVITY
TABLE NO: 1
| Compound No | Zone of inhibition (in mm) | |||||||||
| Quantity in µg/ml | ||||||||||
| B. subtilis | B. pumilis | S. aureus | E. coli | P. vulgaris | ||||||
| 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 | 50 | 100 | |
| 3a | 13 | 22 | 12 | 20 | 13 | 19 | 14 | 20 | 14 | 20 |
| 3b | 15 | 23 | 13 | 22 | 15 | 21 | 17 | 23 | 16 | 24 |
| 3c | 16 | 22 | 15 | 23 | 17 | 20 | 18 | 24 | 17 | 25 |
| 3d | 10 | 18 | 10 | 17 | 09 | 15 | 13 | 18 | 12 | 19 |
| 3e | 11 | 17 | 10 | 16 | 08 | 16 | 12 | 19 | 10 | 19 |
| 3f | 10 | 17 | 10 | 17 | 08 | 15 | 12 | 18 | 10 | 17 |
| 4a | 13 | 18 | 15 | 19 | 15 | 18 | 17 | 19 | 15 | 17 |
| 4b | 10 | 18 | 10 | 17 | 16 | 18 | 17 | 19 | 12 | 14 |
| 4c | 11 | 13 | 11 | 18 | 13 | 16 | 16 | 18 | 13 | 15 |
| 4d | 08 | 12 | 11 | 13 | 14 | 16 | 15 | 19 | 10 | 12 |
| 4e | 09 | 13 | 12 | 15 | 11 | 15 | 16 | 19 | 16 | 18 |
| 4f | 10 | 14 | 12 | 16 | 12 | 15 | 15 | 19 | 15 | 17 |
| Ampicillin | 20 | 25 | 19 | 27 | 19 | 24 | 22 | 28 | 21 | 30 |
ANTIFUNGAL ACTIVITY:TABLE NO: 2
| Compound No | Zone of inhibition (in mm) | |||||
| Quantity in µg/ml | ||||||
| A. niger | C. albicans | R. oryzae | ||||
| 0.05ml | 0.1ml | 0.05ml | 0.1ml | 0.05ml | 0.1ml | |
| 3a | 16 | 22 | 17 | 22 | 15 | 23 |
| 3b | 13 | 16 | 13 | 19 | 10 | 19 |
| 3c | 13 | 17 | 12 | 19 | 10 | 18 |
| 3d | 15 | 19 | 15 | 21 | 12 | 20 |
| 3e | 13 | 17 | 11 | 20 | 10 | 18 |
| 3f | 11 | 15 | 12 | 18 | 12 | 19 |
| 4a | 14 | 18 | 15 | 18 | 13 | 19 |
| 4b | 10 | 13 | 13 | 16 | 10 | 15 |
| 4c | 10 | 14 | 13 | 16 | 11 | 16 |
| 4d | 10 | 13 | 12 | 16 | 10 | 14 |
| 4e | 13 | 17 | 14 | 17 | 12 | 18 |
| 4f | 12 | 16 | 14 | 17 | 11 | 17 |
| Fluconazole | 25 | 28 | 24 | 29 | 22 | 28 |
Discussion
The screening results reveal that all the synthesized novel compounds showed significant antimicrobial activity. Among all the synthesized novel pyrimidine compounds, the novel pyrimidine with 3-nitro substitution (4e) exhibited the effective antibacterial and antifungal activities.
The standard drugs used were Ampicillin and Fluconazole for antibacterial and antifungal activity respectively.
Acknowledgements
The authors are thankful to Dr. Y. Padmanabha Reddy, Principal of RIPER-Anantapur for providing lab facilities and support to complete the research work successfully.
References
- Soliman, K., Ohad, N., Ramadam, N., Maayan, S., Snait, T. and Jacob, V., Bioorg. Med. Chem., 13, 433 (2005).
- Kumar, S.K., Hager, E., Pettit, C., Gurulingappa, H., Davidson, N.E. and Khan, S.R., J. Med. Chem., 46, 2813 (2003).
- Francesco, E., Salvatore, G., Luigi, M. and Massimo, C., Phytochem., 68, 939 (2007).
- Li ming, Z., Hai Shan, J., Liang Peng, S., Hu Ri, P. and Zhe Shan, Q., Bioorg. Med. Chem. Lett., 15, 5027 (2005).
- Nagaraj, A. and Sanjeev Reddy, C., J.Iran.Chem.Soc., 5, 262-267 (2008).
- Feng Jin, Xing Yu Jin., Arch.Pharm.Res., 30, 1359-1367 (2007).
- Shen J.W., Cheng Tsung, L., Lo, Ti. T., Jing, Ru. W., Horng Hueym K., Jih Pyang, W. and Chun Nan, L., Eur. J. Med. Chem., 40, 103 (2005).
- Siva Kumar, P.M., Sreenivasan, S.P., Kumar, V. and Mukesh, D., Bioorg. Med. Chem., Lett., 17, 1695 (2007).
- Frolich, S., Schubert, C., Bienzle, U.C. and Jeneet-siems, K., Journal of Antimicrobial Chemotherapy., 55, 883 (2005).
- Okunade, A.L., Hufford, D.C., Clark, A.L. and Lentz, D., Phytother. Res., 11, 142 (1997).
- Chen, M., Theander, T.G., Christensen, S.B., Zhai,H.L. and Kharazmi, A., Antimicrob. Agents Chemother., 38, 1470 (1994).Pandey, S., Suryawanshi, S.N., Gupta, S. and Srivastava, V.M.L., Eur. J. Med. Chem., 39, 969 (2004).
- Chandra, N., Ramesh, A., Goyal, N., Suryawanshi, S.N., Gupta, S., Eur. J. Med. Chem., 40, 552 (2005).
- Lee, H.U., Kim, B.Y., Ahn, J.B., Kang, S.K., Lee, .J.H., Shin, J.S., Ahn, S.K., Lee, S.J. and Yoon, Z.S., Eur. J. Med. Chem., 40, 862 (2005).
- Agarwal, A., Srivastawa, K., Puri, S.K. and Chauhan, P M.S., Biorg. Med. Chem. Lett, 15, 4923 (2005).
- Shehata, I. A., J. Saudi Chem. Society., 7, 207 (2003).
- Kandeel, M.M. and Omar, A.H., Bull. Faculty Pharmacy, 41, 43 (2003).
- Dora, E.K., Dash, B. and Panda, C.S., J. Het. Chem., 20, 691 (1983).
- Dodson, R.M., Peterson, E.R. and Seyler, J.K., J. Am.Chem. Soc., 72, 3281 (1950).
- Bachstez, M., Ber., 63, 1000 (1930); 64, 2683 (1931).
- Banty A L, The Antimicrobial Susceptibility test; Principle and practice, edited by Illus lea and Febiger, (Philadelphia, Pa USA), 180 (1976).
- Seely H W and Van Demark P J, Microbes in action: A laboratory manual of Microbiology, D.B. Taraporewala Sons and Co, Bombay, 55 (1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.









