Isolation and Characterisation of Oxidation Product of Glycollic Acid by Chromic Acid in Tetrahydrofurane and Tertiary Butyl Alcohol Medium
Manas Rajhans Choubey and Hari Om Pandey*
Department of Chemistry, Ranchi University, Ranchi - 834 008, India.
The complexes of composition [Cr2(OH)2 (C2O4)2 (H2O)4] and [Cr2 (OH)2 (C2O4) (CHO.COO)2 (H2O)4] were isolated by oxidation of glycollic acid with chromic acid in aqueous tetrahydrofuran and tertiary butyl alcohol medium using different chromic acid and glycollic acid ratios. The products isolated were characterised from the elemental analysis, magnetic susceptibility and infrared spectral studies. The substrate having higher oxidation states of chromium could not be isolated in solid form.
KEYWORDS:Chromic acid; Glycollic acid; Oxidised Cr (III) complexes
Download this article as:| Copy the following to cite this article: Choubey M. R, Pandey H. O. Isolation and Characterisation of Oxidation Product of Glycollic Acid by Chromic Acid in Tetrahydrofurane and Tertiary Butyl Alcohol Medium. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Choubey M. R, Pandey H. O. Isolation and Characterisation of Oxidation Product of Glycollic Acid by Chromic Acid in Tetrahydrofurane and Tertiary Butyl Alcohol Medium. Available from: http://www.orientjchem.org/?p=23186 |
Introduction
The kinetics of oxidation of glycollic acid has been studied in detail by chromic acid oxidation methods1-3. The oxidation of glycollic acid to glyoxyllic acid using a microbial cell transformant as catalyst has been reported4. A little work has been done on the isolation and characterization of intermediate complexes formed during the redox reactions. In present investigation we have isolated and characterized the stable complexes formed during the oxidation reactions.
Experimental
The glycollic acid (E. Merck) and chromium trioxide (E. Merck) were used in the reaction. The solvents used tetrahydrofuran (THF) and Tertiary butyl alcohol (TBA) Anal’R grade reagent were used.
The estimation of C and H was obtained from CDRI, Lucknow. Chromium was estimated volumetrically. The FTIR data was recorded on FTIR spectrophotometer from CDRI, Lucknow.
Procedure
1.2 ml of glycollic acid (0.737 M solution) in THF was taken in each case but different amounts of CrO3 in TBA were taken and reaction was carried out in TBA + THF (1:1) medium at 25oC. The amount of CrO3 taken were 1.5g (1M solution) (GTH-1), 1g (0.667 M solution) (GTH – 4) and 0.5g (0.334 M solution) (GTH – 5). The solid compounds were obtained in each case and products formed were collected.
The elemental analysis suggested the proposed formulation (Table – 1) of the chromium complexes formed.
Result and Discussions
The variations of the amount of oxidant (CrO3) for fixed amount of glycollic acid changes the extent of formation of oxalic and glyoxyllic acid contents in the reaction process. The composition of products formed were found between
[Cr2 (C2O4) (CHO – COO)2 – μ – (OH)2] n H2O and
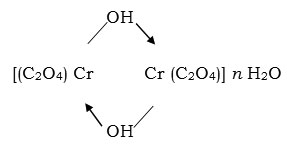
The products formed were partially soluble in cold water and more in hot condition. The solubility of complex in water to some extent can be attributed to interaction of water molecules with complex carboxylate group. It has been found that product formed in excess of CrO3 has composition (Cr2 (C2O4)2 – m – (OH)2 .8 H2O (GTH – 1). The complex formed in appropriate amount of glycollic acid has composition Cr2 (C2O4)2 – μ – (OH)2. 10 H2O (GTH – 4). In lower proportion of oxidant the complex Cr2 (C2O4) – (CHO.COO)2 – μ – (OH)2 .4 H2O (GTH – 5) is formed. The glycollic acid is a weak acid containing one primary alcoholic group and the reaction proceeds by oxidation of alcoholic part (HO – CH2 –) to formyl group (–CHO) and that coordinates with excess of oxidant which further gets oxidised into oxalic acid. The further oxidation of oxalic acid to CO2 was not achieved as evidenced by formation of hydroxoaquo Cr (III) oxalate Cr2 (C2O4)2 m(OH)2.nH2O in good yield.
In presence of less oxidant, CrO3, the glyoxyllic acid (CHO . COOH) and oxalate containing mixed Cr (III) complex has been isolated (GTH – 5).
The presence of aldehydic group in this complex has been confirmed by Tollen’s Reagent test and IR spectral band of (C–H) bending band at 1460.2 cm-1.
The oxidation of HO . CH2 . COOH (glycollic acid) proceeds as shown below :-
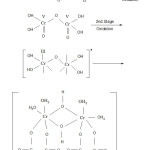 |
Figure 1: Final product Click here to View figure |
If oxidant CrO3 is in less than proper molar ratio, the CHO – COOH remains coordinated as bridging carboxylic acid group as shown :-
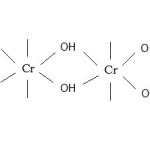 |
Figure 2 Click here to View figure |
 |
Figure 3 and 4 Click here to View figure |
Due to the presence of both species in free glycollic acid5, it shows V(OH) frequency as a broad band between 3600 – 2615 cm-1 region. Due to the predominance of species D, it shows very strong Ketonic group V (CO) stretching band at 1732 cm-1 and V(CO) vibration6 as weak band at 1637 cm-1. The V(CH2) I.R. vibration of glycollic acid enveloped in strong hydrogen bonded V(OH) frequency. The δ(CH2) of methylinic group7 is observed at 1431 cm-1 and V sym (COO) as weak band at 1355 cm-1. The V(C – C) stretching was located in free glycollic acid at 1224 cm-1, while V(C – O) phenolic at 1087 cm-1. The weak I.R. band at 991 cm-1 and 883 cm-1 are attributed to CH2 group wagging and rocking vibrations. The I.R. band at 659 cm-1 and 474 cm-1 are deformation bands.
The oxidation products of glycollic acid display entirely different I.R. vibrations from glycollic acid. The I.R. spectral characteristic of complex is similar to oxalic acid and complex containing hydrogen bonded water molecules. The broad I.R. band between 3600 – 2900 cm-1 supports plenty of water molecules coordinated and attached with hydrogen bonding with oxalic acid carbonyl group. The bond at 2363 cm-1, which is sharp and medium suggests the presence of bridging – OH group
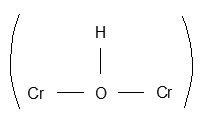
in complexes isolated in each experimental conditions. The broad and very strong I.R. band between 1730 cm-1 and 1550 cm-1 is mixture of d CH2O, V as (C=O) and V(C=O – H – O – H) present in complex. The V s of (C=O) group is also very strong and located at 1404 cm-1 in complexes. The V(C – C) of oxalic acid was observed at 1272 cm-1 and V(C – O) at 1079 cm-1. I.R. band at 935 cm-1 is due to
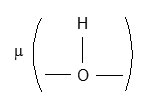
The bands at 805, 765, 611, 502 and 468 cm-1 are attributed to various deformation vibrations in complexes. The strong band at 548 cm-1 can be attributed to V(Cr – OH2) stretching.
Thus, from the evidence of magnetic susceptibility data, elemental analysis and infrared spectral band position, the oxidation of glycollic acid by CrO3 in neutral solvents tetra-hydrofuran and tertiarybutyl alcohol to oxalic acid has been established. The formation of intermediate product glyoxyllic acid (OHC – COOH) and the formation of their chromium (III) complexes have been substantiated.
Table 1 : Elemental analysis of chromium complexes (%)
|
Compound |
Found (Calculated) |
||
|
C |
H |
Cr |
|
| G TH – 1Cr2 (C2O4)2 – m – (OH)2 . 8 H2O |
10.31 (10.38) |
3.73 (3.89) |
23.40 (23.37) |
| G TH – 4Cr2 (C2O4)2 – m – (OH)2 . 10 H2O |
9.80 (9.60) |
4.44 (4.41) |
21.54 (21.68) |
| G TH – 5Cr2 (C2O4)2 (CHO . COO)2 – m – (OH)2.4 H2O |
16.30 (16.07) |
2.71 (2.68) |
23.80 (24.11) |
Table 2 : FTIR absorption bands in cm-1
| Glycollic acid |
3358 |
2615 |
1732 |
1637 |
1431 1224 991 659 |
1355 1087 883 474 |
| GTH – 1 |
3395 |
2363 |
1707 |
1684 |
1407 1089 810 699 |
1272 934 773 544 |
| GTH – 4 |
3406 |
2362 |
1683 |
– |
1407 1080 811 – |
1275 933 773 544 |
| GTH – 5 |
3362 |
2363 |
1590 |
1460 |
1404 1091 809 612 502 |
1272 935 765 549 458 |
References
- Sengupta, Kalyan K.; Chatterjee, A.K.; and Moulik, S.P.; Bulletin of the chemical society of Japan, (1970), Vol. 43, 3841 – 3845.
- Hasan Fariza and Rocek Jan, Journal of the American chemical society, March 19, (1975), 97 : 6.
- Asopa Rachna, Agarwal Saraswati and Banerjee Kalyan K., Proc. Indian Acad. Sci. (Chem. Sci), 4, June (1991), Vol. 103, pp. 563 – 570.
- Anton, David, Leroy; (US) and Dicosimo, Robert; (US), 22 – 7 – (1993), Publication No. wo / 1993 / 0142.
- Silver Stein and Webster, “Spectrometric Identification of organic compounds”, Sixth Edition, (2007), pp 94.
- Nakamoto, K. “Infrared and Raman Spectra of Inorganic and Coordination Compounds” (1988), John Wilky, New York.
- Bellamy, L.J. “The infrared spectra of complex molecules”, Chapman and Hall, London, (1980).

This work is licensed under a Creative Commons Attribution 4.0 International License.









