Evaluation of Adsorption Isotherms of Freundlich, Temkin and Langmuir of Loratadine Drug on Multi-Walled Carbon Nanotube
Mehdi Vadi*, Omid Bazipour and Asadollah Mallekzade
Department of Chemistry, Gachsaran Branch, Islamic Azad University, Gachsaran, Kohgiloyeboyer Ahmad, Iran.
This research has studied equilibrium adsorption isotherms of Loratadine as a drug on carbon nano-tube. And also results of experiments through Freundlich, Temkin and Langmuir models have been studied and it has found different parameters of these models for carbon nano-tube absorbent. According to the error rate for each model, we obtained a good agreement between these data which it showed the best agreement by Langmuir model. With the spectrophotometer (UvVis); it obtained the adsorption diagram based on wavelength for Loratadine. Experimental result showed that by increasing concentrations of Loratadine therefore adsorption rate will increase too. The highest adsorption rate is for 5 ppm concentration of Loratadine.
KEYWORDS:Isotherm; Adsorption; Multi – Walled Carbon nano-tubes; Loratadine
Download this article as:| Copy the following to cite this article: Vadi M, Bazipour O, Mallekzade A. Synthesis 4-(4/-Methylphenyl)-2-Mercaptothiazole Structure With Nmr, Vibrational, Mass Spectroscopy And Dft Studies. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Vadi M, Bazipour O, Mallekzade A. Synthesis 4-(4/-Methylphenyl)-2-Mercaptothiazole Structure With Nmr, Vibrational, Mass Spectroscopy And Dft Studies. Available from: http://www.orientjchem.org/?p=22971 |
Introduction
Carbon nanotubes (CNT) discovery by Iijima in 1991 [1]. Carbon nanotubes (CNTs) are hollow cylinders of graphite carbon atoms. These tubes are on the nanoscale (10-9 m), which is so small that 10,000 of them could fit within the diameter of one human hair. Carbon nano-tubes are a new form of carbon with unique electrical and mechanical properties. They can be considered as the result of folding graphite layers into carbon cylinders. These cylinders may be composed of single shell single wall carbon nanotubes (SWCNTs), or of several shells multi-wall carbon nanotubes (MWCNTs). CNTs can be thought of as a rolled-up sheet of hexagonal ordered graphite formed to give a seamless cylinder. Due to the variety of extraordinary properties exhibited by CNTs, a large number of possible applications have been proposed[2]. Recent discoveries of various forms of CNTs have stimulated research on their applications in diverse fields[3]. The nature of bonding in carbon nanotubes (CNTs) is described by applied quantum chemistry, specifically, orbital hybridization. This chemical bonding is composed entirely of sp2 bonds, similar to those of graphite. This bonding structure, which is stronger than the sp3 bonds found in diamond, provides the molecules with their unique strength. CNTs are the strongest and stiffest materials on earth, in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp² bonds formed between the individual carbon atoms. As for thermal conduction, the CNT surpasses even that of diamond, reaching almost double the value diamond [4]. Carbon nanotubes (CNT) possess many unique characteristics that promise to revolutionize the world of structural materials resulting in significant impact on our capability to build lighter, smaller and higher performance structures for aerospace and many other industrial applications. Based on its unique properties, many applications of CNT have been proposed including quantum wires, tiny electronic devices [5], [6]. Loratadine is a white to off-white powder not soluble in water, but very soluble in acetone, alcohol, and chloroform. It has a molecular weight of 382.89, and empirical formula of C22H23CIN2O2. Loratadine is a second-generation H1 histamine antagonist drug used to treat allergies. Structurally, it is closely related to tricyclic antidepressants, such as imipramine, and is distantly related to the atypical antipsychotic quetiapine. [7]
Material And Methods
Substances
Ethanol (Purity %96) as solvent , Loratadine and adsorbent carbon nano-tubes with a diameter of the outer surface 5-10 nm space 40-600 m2/gr and high purity merck 95% of the company .
Devices used
Spectrophotometer (Uv-Vis) model Lambda 25 perkin elmer
Methods
At first, stock solution of Loratadine was prepared in ethanol (50 mg/l). Then , from stock solution of Loratadine, four standard solutions in concentration of 2,3,4,and 5ppm were prepared . Certain quantities of carbon nano-tube
(0.01 g) have been added as absorbent to 100 mL glasses containing 10 mL Loratadine solution, then it was stirred by mixer for 24 hours (optimal time).After mixing process then the solution should be put into 10000 rpm speed for 20 minutes until the solid and liquid phases are separated. Loratadine concentration was measured before and also after adsorption by spectrophotometer and calibration curve was plotted. All tests have been performed at the lab with the temperature of(C° 2 ± 22).
Results and Discussion
Table (1) shows the amount of Loratadine in the absence of carbon nano-tubes on the solvent ethanol at wavelengths 257(λmax=257nm) on the ethanol solvent. Figure (1) shows the amount of adsorption of Loratadine with different concentrations without the presence of carbon nano-tubes with some walls. As was shown with increasing concentrations of Loratadine adsorption rate increases so that so that we see the most adsorption in 5 ppm by using the figure (1) and Table (1) kit can be calculated the adsorbed Loratadine (0.01 g) on that carbon nano-tubes in table (2) .
Table 1: The adsorption of Loratadine in absent of carbon nanotube
|
adsorption |
concentration |
|
0.396 |
2 |
|
0.557 |
3 |
|
0.679 |
4 |
|
0.847 |
5 |
Table 2: The amount of Loratadine on 0.01 grams of carbon nano-tubes
|
Adsorpation on0.01 gr carbon nanotube ( mg.g-1) |
concentration |
|
1.94 |
2 |
|
2.83 |
3 |
|
3.42 |
4 |
|
4.13 |
5 |
Fig. 1: Adsorption rate without nano-tube Loratadine
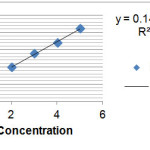 |
Figure 1: Adsorption rate without nano-tube Loratadine Click here to View figure |
Freundlich adsorption isotherms
This model often shows homogeneous absorbent non-adapted hub with experimental data[8,9]. Freundlich adsorption isotherms equation is described below:
qe=kfce1/2 …(1)
q is adsorbate rate in term of mgg-1 and ceq,1/n & kf are adsorbate concentration into solution at the balance moment, from Freundlich constants respectively. These constants are the adsorption severity and adsorption capacity respectively .The following part shows the linear equation:
lnqe=lnkf+1/n Ince …(2
To calculate the constants of Freundlich equation, we draw (lnqe) curve in term of(lnce) according to the presented equation (eq.2) .It is found that if its value (slope of this curve -1/n – is greater therefore the adsorption amount is more too. Here are the related diagram in figure 2 and 1/n- kf parameters at the table 3.
Langmuir model
This model is identical with the energy being adsorbed all the sites on the adsorbent surface obtained [9]. with the following equation
…(3)
Here q is the amount of dissolved adsorbate (mgg-1),Ceq,qe is dissolved concentration in the balance moment b and qm are Langmuir constants.qm is adsorption balance constant and b is also adsorption capacity and a saturated respectively. We can obtain the following relation by rearranging of equation(3):
…(4)
To calculate Lagmuir relation constants; we draw Ce/qe curve in term of Ce which here 1/qm,1/ qmb are slop and width of origin respectively Corresponding graph (Fig. 3) and parameters KL and qm, respectively, represent the maximum energy adsorption and is absorbed in the Table 3.
Temkin isotherm
This model is considered to interact with the adsorbent material by self adsorption which is obtained[8]. With the following equation:
qe = RT/b ln(ACe) …(5)
By Considering RT/b=B A linear form of Temkin isotherm will be as following
Qe=B1lnA+B1lnCe …(6)
In this relation, A (L / mg) is equal bond constant relate to mlent maximum bond energy. B (J / mol) is Temkin isotherm constant and proportional adsorption therm. Adsorption data can be derived form equation (6).
Table 3: Experimental results obtained from the isotherm data
|
Temkin |
Langmuir |
Freundlich |
|
|
R2=0.964 B=0.744 BlnA=4.066 |
R2=0.984 1/qm=0.228 1/qmb=0.021 |
R2=0.968 1/n=0.259 lnkf=1.431 |
Loratadine
|
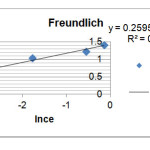 |
Figure 2: Loratadine adsorption diagram based on Freundlich Click here to View figure |
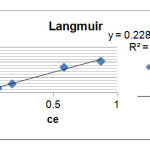 |
Figure 3: Adsorption diagram of Loratadine based on Langmuir model Click here to View figure |
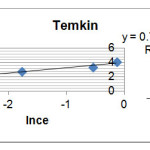 |
Figure 4: Adsorption diagram of Loratadine based on Temkin model Click here to View figure |
Conclusion
In this study we obtained the adsorption isotherms of Loratadine by carbon nanotube. The present research showed that the concentration of carbon nano-tubes of 0.01 grams to adsorb Loratadine has higher efficiency .The parameter that includes Loratadine concentration and type absorber has a strong impact on the efficiency of adsorption. 5ppm concentration reaches its maximum and the constants associated with this isotherm and the correlation coefficient R2 in a linear form in three Tables 3 is given. Based on the correlation coefficient, the Langmuir isotherm has the highest value so Loratadine obeys the Langmuir isotherms.
References
- S.Iijima, Nature 354 (1991), P.56
- PG,Collins P.Avouris Nanotubes for electronics. Sci. A 2000; 283: 38 – 45.
- CNR,Rao AK.Cheetham Science and technology of nano-materials: current status and future prospects. J Mater Chem 11:2887- 94.
- W Hoenlein et al. Integration of carbon nanotubes devices into microelectronics, Mater Res Soc Nanotube-Based Devices Symp. Proceedings. 772: 3 -15.
- T. Hertel, R. Martel and P. Avouris, Manipulation of Individual Carbon Nanotubes and Their Interaction with Surfaces, J. Phys. Chem. B 102: 910915(1998).
- H. Dai, E.W. Wong and C. M. Lieber, Probing Electrical Transport in Nanomaterials: Conductivity of Individual Carbon Nanotubes, Science, 272: 523-526 (1996).
- .Kay GG, Harris AG (July 1999). journal of the British Society for Allergy and Clinical Immunology 29 Suppl 3: 147–50.
- Jeong-yeol yoon,woo -sikkim, Adsoption of BSA on highly carbboxylate microspheres quantitative effects of surface functional groups and interaction forces, J.Colloid and Interface Sci., 177: 613-620-19
- F.martines,A.hernance,protein,Adsorption onto nictofiltration memberance., interaction 254-2612 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









