Dynamic 1h Nmr Studies Along With Theoretical Investigation Around Carbon-Carbon Double Bond In Stable Phosphorus Ylide Containing A 2-Thiazolin-2-Thiol
Sayyed Mostafa Habibi Khorassani*, Malek Taher Maghsoodlou, Ali Ebrahimi, Fatemeh Ghodsi and Razieh Doostmohammadi
Department of Chemistry,The University of Sistan and Balouchestan, P. O. Box 98135-674,Zahedan, Iran
In the present work, dynamic 1H NMR effects were investigated within a particular stable phosphorous ylide involving 4, 5-dihydrothiaZole-2-thiol around the carbon-carbon double bond (OCH3-C?C-PPh3) in the two Z- and E- rotational isomers. Activation parameters including ?H?, ?G?, ?S? were calculated in accord with dynamic 1H NMR data for the rotational process. In addition, theoretical studies were investigated on the same bond using ab initio calculation at HF level and B3LYP method with 6-31G(d, p) basis set. In this case, the Gibbs free energy barrier (?G? = 69.29 and 67.42 kj.mol-1) results were in a good agreement with the experimental data (?G? = 68.71 kj.mol-1) for interchangeable process of rotational isomers between the two 4-Ea and 4-Za
KEYWORDS:Dynamic 1H NMR; Abinitio method; 4; 5-dihydrothiaZole-2-thiol; Triphenylphosphine; Rotational energy barrier
Download this article as:| Copy the following to cite this article: Khorassani S. M. H, Maghsoodlou M. T, Ebrahimi A, Ghodsi F, Doostmohammadi R. Dynamic 1h Nmr Studies Along With Theoretical Investigation Around Carbon-Carbon Double Bond In Stable Phosphorus Ylide Containing A 2-Thiazolin-2-Thiol. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Khorassani S. M. H, Maghsoodlou M. T, Ebrahimi A, Ghodsi F, Doostmohammadi R. Dynamic 1h Nmr Studies Along With Theoretical Investigation Around Carbon-Carbon Double Bond In Stable Phosphorus Ylide Containing A 2-Thiazolin-2-Thiol. Available from: http://www.orientjchem.org/?p=22981 |
Introduction
The synthesis of phosphorus ylides is important in organic chemistry 1-6 because they make stable carbanion. These reactions have gained more use in synthetic organic chemistry 7-11 because of their application in the synthesis of organic products 12-14, especially in the synthesis of naturally occurring products with biological and pharmacological activities 15, 16. These ylides are often prepared by treatment of a phosphonium salt with a base, and phosphonium salts are usually obtained from the phosphine and an alkyl halide. Some ylides exhibited dynamic 1HNMR effect that affords good information regarding the interchangeable process of rotational isomers that provide important kinetic data 17-26. In previous works, dynamic 1H NMR effects were reported for dimethyl-2-(4, 5 dihydrotiaZolin-N-yl)-3-(triethoxyposphoranylidene)-butanedioate 27. Now, we describe dynamic 1H NMR effect for reaction between dimethyl acetylendicarboxylate 2 and 2- thiaZolin-2-thiol 3 in the presence of triphenylphosphine 1 for generation of phosphorous ylide 4a involving the two Z – and E-rotational isomers . Synthesis of this reaction has been earlier reported 28 (Figure1-i)
Material and methods
Triphenylphosphine, dialkyl acetylenedicarboxylates and 4, 5-dihydrothiaZole-2-thiol were
purchased from Fluka and without further purification. Quantum mechanical calculation has
been performed using the Gaussian 03 package 29 at the HF 30, 31 and B3LYP 32, 33 methods with 6-31G(d,p) basis set. The 1H, 13C and 31P NMR spectra were recorded on a Bruker DRX–500 AVANCE instrument with CDCl as solvent at 500.1, 125.8 and 202.4 MHZ, respectively.
Results and discussions
The reaction between dimethyl acetylenedicarboxylate 2 and 4, 5-dihydrothiaZole-2-thiol 3 in the presence of triphenylphosphine 1 was completed over a few hours (or after 8 hours) at ambient temperature in ethyl acetate as solvent. The 1H and 13C NMR spectra of the product indicated formation of 4a. The structure of compound 4a was verified from elemental analyses data, mass, IR, 1H, 13C and also 31P NMR spectra data. The mass spectra of 4a displayed molecular ion peak at m/Z= 523. The 1H and 31P spectra of stable ylide 4a are consistent with the presence of the two rotational isomer forms (Z-4a and E-4a, Figure 1-j), the C2-C3 has a partial double bond character, due to the conjugation with adjacent carbonyl group of CO2Me and C=p+(PPh)3, as shown in Figure 1-j.
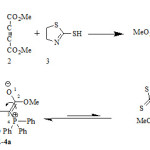 |
Figure 1. i) Reaction between dimethyl acetylendicarboxylate 2 and 4, 5-dihydrothiaZole-2-thiol 3 in the presence of triphenylphosphine 1 for preparation of phosphorus ylide 4a.j) Interchangeable process of rotational isomers in the two 4-Ea and 4-Za isomers Click here to View figure |
The rotation around the carbon-carbon double bond (H3COO-C=C-PPh3) in the Z-4a and the E-4a geometrical isomers is slow on the NMR time scale at ambient temperature. Thus, this provides a mixture of the two Z-4a and E-4a geometrical isomers. The 1H NMR spectra of the phosphorous ylide 4a exhibit two doublets for the methine proton(H-C-C-P, 3JPH= 17.5HZ) at d= 5.5 and 5.55 ppm (Figure 2) and two singlets arising from methoxy groups at 3.53 and 3.75 ppm and also at 3.13 and 3.78 ppm for the two minor (E-4a) and major (Z-4a) geometrical isomers, respectively (Figure 3). Effects of dynamic 1HNMR around the partial carbon-carbon double bond will be argued next. On the basis of 1H NMR spectra at high temperature, two sharp signals was observed for the methoxy groups at 40°C (313 K), due to a fast rotational around the carbon-carbon double bond (Figure 3).Investigation of such behavior in the phosphorus ylide 4a at variable temperatures allowed us to calculate the Gibbs free energy barrier (∆G≠) for the restricted rotation around the carbon-carbon double bond in 4a (Table 1).
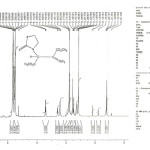 |
Figure 2 1H NMR spectra of compound 4a. Click here to View figure |
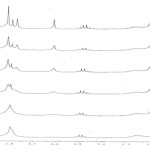 |
Figure 3. Dynamic 1H NMR effects at variant for methoxy groups in compound 4a |
Using the expression kc= π∆υ/√2, the first–order rate constant was calculated for this rotation at 40 ºC. Application of the absolute rate theory with a transmission coefficient (k) of one, gave a Gibbs free energy barrier. These values are reported in Table 1. Effect of temperature on the rate constant was investigated on the basis of measurement of different chemical shifts in a series of other separate experiments. The results were too small so that the changes in first-order rate constant and Gibbs free energy barrier were negligible in comparison with the results obtained at 40°C (313K).
Table 1. Selected 1H chemical shifts (at 500.1 MHZ, in ppm, Me4Si) for ylide 4a along with activation parameters in CDCl3, for restricted rotational process around the carbon-carbon double bond P(Ph)3 P–CC–OMe) in ylide 4a and speculative ylide 5a at 298 K (room temperature) and experimental temperature (313 K).
|
|
∆S# (J⁄mol) |
∆H# (kJ⁄mol) |
∆G# (kJ/mol) |
∆υ (HZ) |
δ (ppm) |
Tc (K) |
|
Experimental a |
28.66 |
74.67 |
68.71 |
9.41 |
5.55, 5.5 |
313 |
|
Theoretical b |
-11.04 |
65.88 |
69.16 |
– |
– |
298 |
|
Theoretical c |
-11.31 |
65.75 |
69.29 |
– |
– |
313 |
|
Theoretical d |
-23.32 |
60.12 |
67.42 |
– |
– |
313 |
|
Theoretical e |
-15.97 |
68.39 |
73.15 |
– |
– |
298 |
a Experimental Data obtained from the 1H NMR study 28
![]()
b Theoretic data obtained using ab initio calculation at HF/6-31G(d,p) level of theory for a synthesiZed ylide 4a at 298K
![]()
c Theoretical data obtained using ab initio calculation at HF/6-31G(d,p) level of theory at experimental temperature (313K) for synthesiZed
ylide 4a.
d Theoretical data obtained using ab initio calculation at B3LYP method with 6-31G(d,p) basis set at experimental temperature (313K)for a synthesiZed ylide 4a.
e Theoretical data obtained using ab initio calculation at HF/6-31G(d,p) level of theory for a speculative ylide 5a at 298K.
In order to determine theoretical rotational energy barrier around the CC bond in ylide 4a (Figure 1-j), first their structures were optimiZed at HF/6-31G level 30, 30 of theory by Gaussian 03 program package 29 and relative energy between the two isomers calculated at HF/6-31G(d, p) level and B3LYP method with 6-311++G(d, p) basis set. The results are given in Table 2.
Table 2. The relative energy (kj/mol) between the two isomers of ylid 4a, obtained at HF/6-31G(d,p) and B3LYP method with 6-311++G(d,p) basis set.
|
B3LYP |
HF |
isomer |
|
40.41 |
48.93 |
E |
|
0 |
0 |
Z |
Then, relative energy was plotted versus dihedral angle O1C2C3P4 (Figure 4) as shown in Figure 5. The plots in Figure 5 and 6 were obtained by scanning method at HF/6-31G(d, p) level of theory by Gaussian 03 and each point in both figures were fully optimiZed. A repetition of this behavior was carried out for a speculative ylide 5a (Figure 4) in comparison with the synthesiZed ylide 4a. In a speculative ylide 5a, sulfur atom in conjugate base of N-H acid has been joined to C5 in phosphorus ylide, while in ylide 4a nitrogen atom of conjugate base has been joined to C5 atom. The results shown in Figure 6 and Table 1.
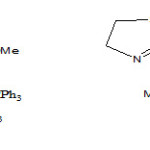 |
Figure 4. The performance of O1C2C3P4 dihedral angle in a synthesiZed phosphorus ylide 4a and in a speculative ylide 5a |
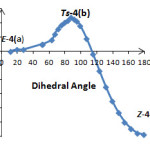 |
Table 1: Click here to View figure |
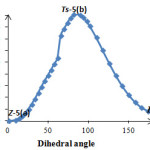 |
Figure 6. Relative energy versus dihedral angel O1C2C3P4 in a speculative phosphorus ylide 5a Click here to View figure |
The corresponding structures, with respect to all points (a, b and c) in Figure 5 were drawn in Figure 7. Frequency calculations were employed for all transition and stationary points (TS, E–4a and Z-4a) and the results are reported in Table 3. First negative frequency value could be considered as a strong proof for confirming of TS structure.
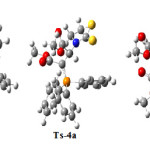 |
Figure 7. Structures corresponding to a, b and c points with respect to Figure 1-j |
Table 3.
Frequency data obtained for all TS, E-4a and Z-4a structures in the ylide 4a.
|
Isomer |
First Frequency |
|
|
E-4a TS Z-4a |
20.68 – 66.16 13.74 |
On the basis of theoretical calculations, for synthesiZed ylide 4a, and data obtained from the Figures 5 and 6, the Gibbs free energy barrier (ΔG≠), ΔH≠ and ΔS≠ calculated around the carbon-carbon double bond for both the speculative and synthesiZed ylide. As can be seen from Table 3; A) there is, relatively, a good agreement between the experimental data (a) and theoretical data (b, c) at 298K and experimental temperature (313). B) there is also a good consistent at both HF level and B3LYP method with 6-31G(d, p) basis set (c, d) with experimental data (a). In addition, theoretical Gibbs free energy barrier for a speculative ylide 5a (Table 1, e) is higher than both Gibbs free energy obtained from experimental data (Table 1, a) and theoretical data (Table 1, b, c and d) in a synthesiZed ylide 4a. These results indicate that why the ylide 4a is practically generated easier than the speculative ylide 5a.
Conclusion
The theoretical results on the basis of mechanical calculations, obtained from ab initio calculation at HF level and B3LYP method with 6-31G (d, p) basis set indicated that activation parameters (ΔG≠ = 69.29 and 67.42 kj.mol-1, respectively) for interchangeable process between the two rotational isomers (4-E and 4-Z) were relatively consistent with the experimental dynamic 1H NMR data (ΔG≠ = 68.71 kj.mol-1). In addition, the results show that generation of the synthesiZed ylide, 4a is more possible than the speculative ylide, 5a because, of the less free Gibbs energy barrier.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of the University of Sistan & Baluchestan.
References
- Hudson H. R., Vol. 1 Wiley: New York,. 386, (1990).
- Habibi-Khorassani S.M., Maghsoodlou M. T., Ebrahimi A., Zakarianejad M., Fattahi M. J. Solution Chem., 36, 1117, (2007).
- Cadogan J. I. G. Academic Press: New York, (1979).
- Maghsoodlou M. T., HaZeri N., Habibi-Khorassani S. M., Kakaei R., Nassiri M., Phosphorus, Sulfur Silicon Relat. Elem., 181, 25, (2006).
- Habibi-khorassani S.M., Ebrahimi A., Maghsoodlou M.T., Saravani H., ZakarianeZhad M., GhahramanineZhat M., KaZemian M.A., Nassiri M., khajehali Z. Reaction Kinetics Mech., 34,. 261, (2009).
- Corbridge D. E. C. Phosphorus:, 5th ed.; Elsevier: Amsterdam, (1995).
- . Maghsoodlou M.T., HaZeri N., Habibi Khorassani S. M., Nassiri, M., Marandi,G., ShahZadeh, A. G., BijanZadeh, H. R., Phosphorus, Sulfur, Silicon Relat Elem, 181,. 1117, (2006).
- . Nair V. C. , Rajesh A. U. Vinod S. Bindu, A. R. Sreekanth J. S. Acc Chem Res., 36,. 899, (2003).
- . Maghsoodlou M.T., HaZeri. N., Habibi Khorassani S. M., Heydari R., Nassiri M., Moeeni Z., Nirumand u., Eskandari Torbaghan. Z., Phosphorus, Sulfur, Silicon Relat Elem., 181, 865, (2006).
- . Yavari I., Maghsoodlou M. T., Tetrahedron Letters, 39, 4579, (1998).
- . Maghsoodlou M. T., HaZeri. N., Habibi-Khorassani S. M., Afshari G., Nassiri. M. J. Chem. Res, 727, (2005).
- . Maghsoodlou M. T., Heydari. R., Habibi-Khorassani. S. M., Rofouei M. K., Nassiri M., Mosaddegh E., Hassankhani A., Journal of Sulfur Chemistry, 27,. 341, (2006).
- . Habibi-KhorassaniS. M., Maghsoodlou M. T., Nassiri M., ZakarianeZhad M., Fattahi M. Arkivoc, xvi,. 168, (2006).
- Habibi-Khorassani. S. M., Ebrahimi A., Maghsoodlou M. T., Rostami Charati F., KaZemian M, A., Karimi P, Phosphorus, Sulfur Silicon Relat. Elem.,. 559, (2010).
- Maghsoodlou M. T., Heydari R., HaZeri N., Habibi-Khorasani S. M.; Nassiri M., KaZemian M.A., SalehZadeh J., HajiZadeh M., GhasemZadeh M., Mosaddegh E, Biomedical and Pharmacological Journal, 1,. 51,(2008).
- .. Habibi Khorassani S.M., Maghsoodlou M.T., Ebrahimi A., ZakarianeZhad M.,. MohamadZadeh p, Shahraki M., Oriental Journal of chemistry, 24, 73. (2008)
- . Habibi-Khorasani S. M.; Ebrahimi A.; Maghsoodlou M. T.; Same-Salari S.; Nasiri S., Ghasempour H., Magnetic Resonance in Chemistry, 49(5), 213, (2011).
- . Kabiri R.; HaZeri N.; Habibi-Khorasani S. M.; Maghsoodlou M. T.; Ebrahimi A., Saghatforoush L.; Marandi G., RaZmjoob Z., ARKIVOC, 17. 12, (2008).
- . Habibi-Khorasani S. M., Maghsoodlou M. T., Ebrahimi A., Farahani Vasheghani F., Mosaddeg E.; KaZemain M.A., TETRAHEDRON LETTERS, 50,. 3621, (2009).
- Maghsoodlou M. T.; Marandi G., Habibi-Khorasani S. M., Saghatforoush L., Aminkhani A.; Kabiri R, Indian Journal of Chemistry, 47B,. 1151, (2008).
- . HaZeri N., Habibi-Khorasani S. M., Maghsoodlou M. T.,Marandi G., Nassiri M., ShahZadeh, A, journal of Chem.Res., 4,. 215 (3), (2006).
- . CervinkaO. In Roppoport, Z. Eds., Wiley: New York, Part 1.PP. 219, (1994).
- . Anet F. A. L.; Anet R.; Cotton F. A.; Jackman, L. M. Eds., Academic Press: New York, Chapter 8,(1979).
- . Oki M., Eds., VCH: Weinheim, (1985).
- . Ziyaadini M.; Maghsoodlou M. T., HaZeri. N., Habibi-Khorassani S. M., Hetroatom Chem, 23, 131, (2011).
- 26. Maghsoodlou M. T., Shaterian H. R., Marandi. G., Shahraki Poor F., SalehZadeh. J. , Arkivoc (xiii),. 218, (2008).
- 27. . HabibiKhorassani S. M., Ansari Fard M., Mohammadi M., Aghdaei E., Shahraki M., HadiZadeh M., HaZeri N. Jordan of chemistry, 7, 23, (2012).
- . Maghsoodlou M. T.;HaZeriN.; Habibi-Khorasani S. M.; Nassiri M.; Marandi G.; Afshari G.; Niroumand U. Sulfur chemistry, 26,. 261, (2005).
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, Jr., J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; YaZyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; ZakrZewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; OrtiZ, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; PiskorZ, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; GonZaleZ, C.; and Pople, J. A.; Gaussian, Inc., Wallingford CT, (2004).
- Petersson G. A., Al-Laham M. A. J Chem Phys, 94,6081, (1991)
- . Petersson G. A., Bennett A., Tensfeldt T. G., Al-Laham M.A., Shirley WA, MantZaris J J Chem Phys, 89, 2193.(1988), Becke A. D. Phys Rev A, 38, 3098, (1988)
- Lee C., Yang W., Parr R. G. Phys Rev B, 37,785, (1988)
- Becke, A. D. Phys Rev A, 38, 3098, (1988)

This work is licensed under a Creative Commons Attribution 4.0 International License.









