Comparative Study of Adsorption Isotherms two Nonsteroidal Anti-Inflammatory Drugs(Nsaids), Acetaminophen and Diclofenac on Carbon Nanotube
Mehdi Vadi* and Asma Omidi
Department of Chemistry, Gachsaran Branch, University of Gachsaran, Kohgiloyeh va Boyerahmad, Iran
We have studied the interaction of Acetaminophen and Diclofenac solutions, on multi ncarbon nanotube. After investigated comparative study and assigned to either Acetaminophen or Diclofenac adsorption isotherms. The adsorption equilibrium isotherms were fitted by Freundlich, Langmuir, and Temkin models. It was found that the Temkin model described the adsorption process better than other two isotherm models. The amount of NSAIDs (Acetaminophen and diclofenac)adsorbed on carbon nanotube surface increased with the increase of the initial NSAIDs concentration. The results show that diclofenac has most amount adsorption rate of MWCNT.
KEYWORDS:Adsorption; Isotherms; Non-steroidal; Carbon nanotube
Download this article as:| Copy the following to cite this article: Vadi M, Omidi A. Comparative Study of Adsorption Isotherms two Nonsteroidal Anti-Inflammatory Drugs(Nsaids), Acetaminophen and Diclofenac on Carbon Nanotube. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Vadi M, Omidi A. Comparative Study of Adsorption Isotherms two Nonsteroidal Anti-Inflammatory Drugs(Nsaids), Acetaminophen and Diclofenac on Carbon Nanotube. Available from: http://www.orientjchem.org/?p=22977 |
Introduction
Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1,[1] significantly larger than for any other material. These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, electronics, optics and other fields of materials science and technology. In particular, owing to their extraordinary thermal conductivity and mechanical and electrical properties, carbon nanotubes find applications as additives to various structural materials. For instance, nanotubes form only a tiny portion of the material(s) in (primarily carbon fiber) baseball bats, golf clubs, or car parts.[2] Nanotubes are members of the fullerene structural family, which also includes the spherical buckyballs, and the ends of a nanotube may be capped with a hemisphere of the buckyball structure. Their name is derived from their long, hollow structure with the walls formed by one-atom-thick sheets of carbon, called graphene. These sheets are rolled at specific and discrete (“chiral”) angles, and the combination of the rolling angle and radius decides the nanotube properties; for example, whether the individual nanotube shell is a metal or semiconductor. Nanotubes are categorized as single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). Individual nanotubes naturally align themselves into “ropes” held together by van der Waals forces, more specifically, pi-stacking. Applied quantum chemistry, specifically, orbital hybridization best describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite. These bonds, which are stronger than the sp3 bonds found in alkanes and diamond, provide nanotubes with their unique strength.Multi-walled nanotubes (MWNT) consist of multiple rolled layers (concentric tubes) of graphene. There are two models that can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, e.g., a (0,8) single-walled nanotube (SWNT) within a larger (0,17) single-walled nanotube. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 3.4 Å. The Russian Doll structure is observed more commonly. Its individual shells can be described as SWNTs, which can be metallic or semiconducting. Because of statistical probability and restrictions on the relative diameters of the individual tubes, one of the shells, and thus the whole MWNT, is usually a zero-gap metal. Double-walled carbon nanotubes (DWNT) form a special class of nanotubes because their morphology and properties are similar to those of SWNT but their resistance to chemicals is significantly improved. This is especially important when functionalization is required (this means grafting of chemical functions at the surface of the nanotubes) to add new properties to the CNT. In the case of SWNT, covalent functionalization will break some C=C double bonds, leaving “holes” in the structure on the nanotube and, thus, modifying both its mechanical and electrical properties. In the case of DWNT, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003[3] by the CCVD technique, from the selective reduction of oxide solutions in methane and hydrogen. The telescopic motion ability of inner shells [4] and their unique mechanical properties[5] permit to use multi-walled nanotubes as main movable arms in coming nanomechanical devices. Retraction force that occurs to telescopic motion caused by the Lennard-Jones interaction between shells and its value is about 1.5 nN.[6]
Nonsteroidal anti-inflammatory drugs, usually abbreviated to NSAIDs but also referred to as nonsteroidal anti-inflammatory agents/analgesics (NSAIAs) or non steroidal anti-inflammatory medicines (NSAIMs) are drugs that provide analgesic and antipyretic (fever-reducing) effects, and, in higher doses, anti-inflammatory effects.The term “nonsteroidal” distinguishes these drugs from steroids, which, among a broad range of other effects, have a similar eicosanoid-depressing, anti-inflammatory action. As analgesics, NSAIDs are unusual in that they are non-narcotic.The most prominent members of this group of drugs are aspirin, ibuprofen, and naproxen, all of which are available over the counter in most countries.[7][8] NSAIDs are usually indicated for the treatment of acute or chronic conditions where pain and inflammation are present. Research continues into their potential for prevention of colorectal cancer, and treatment of other conditions, such as cancer and cardiovascular disease .NSAIDs are generally indicated for the symptomatic relief of the following conditions:[9]Rheumatoid arthritis[10] Osteoarthritis An analgesic (also known as a painkiller) is any member of the group of drugs used to relieve pain (achieve analgesia). The word analgesic derives from Greek an- (“without”) and algos (“pain”). Analgesic drugs act in various ways on the peripheral and central nervous systems; they include paracetamol (para acetylaminophenol, also known in the US as acetaminophen), the non-steroidal anti-inflammatory drugs (NSAIDs) such as the salicylates, and opioid drugs such as morphine and opium. They are distinct from anesthetics, which reversibly eliminate sensation. In choosing analgesics, the severity and response to other medication determines the choice of agent; the who pain ladder, originally developed in cancer-related pain, is widely applied to find suitable drugs in a stepwise manner.[11] The analgesic choice is also determined by the type of pain: for neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants.[12] The inflammation response of our bodies is a key part of the healing process. Using NSAIDs to reduce the inflammation has been shown to impair healing in different tissue types: Muscles. [13]. A 2001 study showed that Ibuprofen and Acetaminophen reduce muscle growth after eccentric exercise. Another study[14] on muscle damage and NSAIDs showed impaired recovery in the early stages of healing. There was some increased protein synthesis with NSAIDs in latter stages of healing, but the muscles were still weaker 28 days after injury. Other studies[15][16] have shown that four days after injury, NSAIDs resulted in very little muscle regeneration compared with no drugs. To quote from the study “Given that NSAID administration was discontinued after 14 days yet affected load-to-failure eight weeks following repair, it appears that inhibition of the early events in the inflammatory cascade has a lasting negative effect on tendon-to-bone healing A study [17] in 2004 declared ” Nonsteroidal anti-inflammatory drugs continue to be prescribed as analgesics for patients with healing fractures even though these drugs diminish bone formation, healing, and remodeling”.
Diclofenac Formula
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) taken to reduce inflammation and as an analgesic reducing pain in certain conditions .designated chemically as 2-(2-(2,6-dichlorophenylamino)phenyl)acetic acid .The molecular weight is 296.148 g/mol. Its molecular formula is C14H11Cl2NO2 . The structural formula is:
Acetaminophen Formula
Acetaminophen is a nonsteroidal anti-inflammatory drug (NSAID).designated chemically as N-(4-hydroxyphenyl) acetamide , Its molecular weight is 151.17. Its empirical formula is C8H9NO2 and its structural formula is:
Experimental
Material
We used the carbon nanotube with 95%pure degree, production of neutrino Company. Acetaminophen and diclofenac with 99% pure degree production of Pharmaceutical companies Aboureihan
Apparatus
In this survey the spectrophotometer (Jenway6505, Model, England), magnetic stirrer
(Heidolph, Mr3001 Model), Analytical balance (Sartorius Model), Filter paper (Albet), were used.
Adsorption experiments
In order to provide Acetaminophen and Naproxen solution, twice distilled water was used .multi walled carbon nanotube was used as an adsorbent. first 100ppm of acetaminophen and Diclofenac was provided using this sample, some solutions with different concentrations of 5 to 11 mg/lit of pure Acetaminophen and Diclofenac were prepared specific amount of carbon nanotube (0.01gr) was added to flasks containing Acetaminophen and Diclofenac ,as an adsorbent .it was stirred , using a magnetic stirrer for 10 minutes. Then liquid and solid phase were separated by means of a filter paper. the concentration of Acetaminophen and Diclofenac was measured by using on spectrophotometer tool adsorption rate, gained for NSAIDs .all test have been performed at the lab with the temperature.
Adsorption isotherms
Equilibrium study on adsorption provides information on the capacity of the adsorbent. An adsorption isotherm is characterized by certain constant values, which express the surface properties and affinity of the adsorbent and can also be used to compare the adsorptive capacities of the adsorbent for different pollutants. Equilibrium data can be analyzed using commonly known adsorption systems. Several mathematical models can be used to describe experimental data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment. data of adsorption isotherms. The Freundlich, Langmuir and Temkin models are employed to analysis adsorption occurred in the experiment.
Langmuir model
The Langmuir adsorption model [28] is the most common model used to quantify the amount of adsorbate on an adsorbent as a function of partial pressure or concentration at a given temperature. This equation expressed by relation.
n this equation, (mg. ) is the solution was adsorbed the surface and is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and
( mg. ) is solution in equilibrium state.
Freundlich model
The Freundlich equation or Freundlich adsorption isotherm is an adsorption isotherm, which is a curve relating the concentration of a solute on the surface of an adsorbent, to the concentration of the solute in the liquid with which it is in contact. In 1909, Freundlich gave an empirical expression representing the isothermal variation of Adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with pressure. This equation is known as Freundlich Adsorptio nIsotherm or Freundlich Adsorption equation. This model is specified with equation.
In this equation, (mg. ) is amount of absorbed material in absorbent surface, K, n in arrangement are adsorption capacity and adsorption intensification.
Temkin model
The Temkin model is linearly represented as equation (3) and generally applied in the form:
Where A and B are the Temkin isotherm constant (L/g) and heat of sorption (J/mol)
respectively. R is the gas constant (J/mol/k), b is the Temkin isotherm constant linked to the energy parameter, B, as shown on equation:
T is the absolute temperature in Kelvin.
Results and Discussion
Adsorption isotherms
The Langmuir, Freundlich and Temkin isotherms of the adsorption process of NSAIDs on CNT are shown in figures 1 to 3 and calculated parameters of these models are shown in Table 1. It
was observed that the experimental data were well represented by Langmuir Freundlich and Temkin models.
Table 1: Parameters of Langmuir, Freundlich and Temkin isotherms of the Acetaminophen and Diclofenac on CNT
| Langmuir | Freundlich | Temkin | |||||||||||
| b | q | n | k | A | b | B | |||||||
| Acetaminophen
|
e | 58.82 | 0.10 | 1.07
|
0.92 | e | 4.26 | 0.96 | |||||
| Diclofenac
|
e | 34.48 | 0.42 | 1.16 | 0.94 | e | 4.67 | 0.97 | |||||
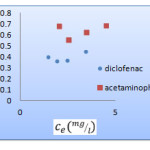 |
Figure 1:Langmuir isotherm of Acetaminophen
and diclofenac on CNT |
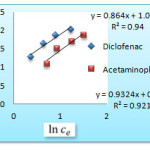 |
Figure 2: Freundlich isotherm of Acetaminophenand diclofenac on CNT Click here to View figure |
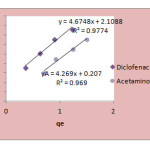 |
Figure 3:Temkin isotherm of Acetaminophen and diclofenac on CNT
|
Conclusions
We have studied the interaction of Acetaminophen and diclofenac with as-prepared and purified multi-walled carbon nanotubes (MWCNTs). Optical spectroscopic data Uv/Vis showed that purified carbon nanotubes interact with Acetaminophen and diclofenac. These experimental results are confirmed by first principles calculation that predict a very weak adsorption process through p–p interaction instead of through the free electron pair of the N and Cl .Spectroscopic studies showed that the interaction of Acetaminophen and diclofenac with carbon nanotubes is weak, in agreement with theoretical predictions based on ab initio calculations that indicate that for both metallic and semiconducting nanotubes the interaction occurs via p–p orbital interaction between the Acetaminophen and diclofenac aromatic rings and the C-ring of the MWCNTs, instead of through the free electron pair of the N and Cl. In this research, adsorption some of Non-steroidal anti-inflammatory drugs eye (Acetaminophen and diclofenac) on multiwall carbon nanotube from aqueous solution were studied. The results show that the amount of Acetaminophen and diclofenac adsorbed on carbon nanotube surface increased with the increase of the initial NSAIDs concentration. The results show that diclofenac has most amount adsorption rate of MWCNT because it can be attributed to lower the barrier of space in this material. The experimental results show that it was found that the Temkin model described the adsorption process better than other two isotherm models.
References
- Wang, X.; Li, Qunqing; Xie, Jing; Jin, Zhong; Wang, Jinyong; Li, Yan; Jiang, Kaili; Fan, Shoushan (2009). “Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates”. Nano Letters 9 (9): 3137–3141. DOI:10.1021/nl901260b. PMID 19650638.
- Gullapalli, S.; Wong, M.S. (2011). “Nanotechnology: A Guide to Nano-Objects”. Chemical Engineering Progress 107 (5): 28–32.
- Flahaut, E.; Bacsa, Revathi; Peigney, Alain; Laurent, Christophe (2003). “Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes”. Chemical Communications 12 (12): 1442–1443. DOI:10.1039/b301514a. PMID 12841282.
- Cumings, J.; Zettl, A. (2000). “Low-Friction Nanoscale Linear Bearing Realized from Multiwall Carbon Nanotubes”. Science 289 (5479): 602–604. DOI:10.1126/science.289.5479.602. PMID 10915618
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. (1996). “Exceptionally high Young’s modulus observed for individual carbon nanotubes”. Nature 381 (6584): 678–680. DOI:10.1038/381678a0
- Zavalniuk, V.; Marchenko, S. (2011). “Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes”. Low Temperature Physics 37 (4). 337. arXiv:0903.2461. DOI:10.1063/1.3592692.
- Stuart J. Warden, PT, PhD, FACSM (April 2010). “Prophylactic Use of NSAIDs by Athletes:A Risk/Benefit Assessment”. The Physician and SportsMedicine. 38 (1): 132–138. DOI:10.3810/psm.2010.04.1770. PMID 20424410.
- Some Common Medications That Contain Acetaminophen, Acetaminophen is also a common NSAID.
- Simone Rossi, ed. (2006). Australian medicines handbook 2006. Adelaide: Australian Medicines Handbook Pty Ltd. ISBN 0-9757919-2-3.
- Gøtzsche, Pc (March 1989). “Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis”. Controlled clinical trials 10 (1): 31–56. DOI:10.1016/0197-2456(89)90017-2. ISSN 0197-2456. PMID 2702836.
- Anonymous (1990). Cancer pain relief and palliative care; report of a WHO expert committee. World Health Organization Technical Report Series, 804. Geneva, Switzerland: World Health Organization. pp. 1–75. ISBN 92-4-120804-X.
- Dworkin RH, Backonja M, Rowbotham MC, et al. (2003). “Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations”. Arch. Neurol. 60 (11): 1524–34. DOI:10.1001/archneur.60.11.1524. PMID 14623723.
- Skeletal Muscle PGF2αand PGE2 in Response to Eccentric Resistance Exercise: Influence of Ibuprofen and Acetaminophen http://jcem.endojournals.org/content/86/10/5067.long
- An In Vitro Investigation Into the Effects of Repetitive Motion and Nonsteroidal Antiinflammatory Medication on Human Tendon Fibroblasts http://ajs.sagepub.com/content/23/1/119
- Cost-conscious prescribing of nonsteroidal anti-in… [Arch Intern Med. 1992] – PubMed result http://www.ncbi.nlm.nih.gov/pubmed/1417372
- Sports Injuries – NSAIDs: Why We Do Not Recommend Them http://www.caringmedical.com/sports_injury/nsaids.asp
- Effects of Nonsteroidal Anti-Inflammatory Drugs on Bone Formation and Soft-Tissue Healing — Dahners and Mullis 12 (3): 139 — Journal of the American Academy of Orthopaedic Surgeons http://www.jaaos.org/cgi/content/abstract/12/3/139

This work is licensed under a Creative Commons Attribution 4.0 International License.









