Ammonium Chloride: An Effective Catalyst For The One-Pot Synthesis Of 2,4,5-Trisubstituted Imidazoles
Behrooz Maleki1*, Hossein Keshvari1 and Ali Mohammadi2*
1Department of Chemistry, Hakim Sabzevari University, Sabzevar, Iran. 2Department of Chemistry, Faculty of Sciences, Ferdowsi University, Mashhad, Iran.
An efficient synthesis of 2,4,5-trisubstituted imidazoles is achieved by three component cyclocondensation of 1,2-dicarbonyl compound, aldehyde and ammonium acetate using Ammonium chloride (NH4Cl) as a catalyst. The key advantages of this process are high yields, cost effectiveness of catalyst, easy work-up and purification of products by non-chromatographic method.
KEYWORDS:2, 4, 5-Trisubstituted imidazoles; Solvent-free synthesis; Multicomponent reaction; Ammonium chloride; Heterogeneous catalysts
Download this article as:| Copy the following to cite this article: Maleki B, Keshvari H, Mohammadi A. Ammonium Chloride: An Effective Catalyst for the One-Pot Synthesis of 2,4,5-Trisubstituted Imidazoles. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Maleki B, Keshvari H, Mohammadi A. Ammonium Chloride: An Effective Catalyst for the One-Pot Synthesis of 2,4,5-Trisubstituted Imidazoles. Available from: http://www.orientjchem.org/?p=22935 |
Introduction
Multicomponent reactions (MCRs) have drawn great interest enjoying an outstanding status in modern organic synthesis and medicinal chemistry because they are one-pot processes bringing together three or more components and show high atom economy and high selectivity [1, 2]. MCRs have great contribution in convergent synthesis of complex and important organic molecules from simple and readily available starting materials, and have emerged as powerful tools for drug discovery [3, 4]. The imidazole nucleus is a fertile source of biologically important molecules. Compounds containing imidazole moiety have many pharmacological properties and play important roles in biochemical processes. They are well known as inhibitors of P38MAP kinase, fungicides, herbicides, anti-inflammatory agents, antithrombotic agents, plant growth regulators and therapeutic agents. In addition, they are used in photography as photosensitive compounds. Some substituted triarylimidazoles are selective antagonists of the glucagons receptor and inhibitors of IL-1 biosynthesis [5]. Radziszewski and Jaap proposed the first synthesis of the imidazole core in 1882, starting from 1,2-dicarbonyl compounds, aldehydes and ammonia to obtain 2,4,5-triphenylimidazole [6, 7]. There are several methods for the synthesis of 2,4,5-triarylimidazoles using ZrCl4, zeolites HY/silica gel, NaHSO3, sulphanilic acid, iodine, ceric ammonium nitrate, oxalic acid, ionic liquids and also by microwave irradiation using acetic acid. Each of the above methods for this reaction has its own merits, while some of the methods are plagued by the limitations of poor yield, longer reaction time, difficult work-up and effluent pollution [5]. Therefore, the development of a new mild method to overcome these disadvantages still remains a challenge for organic chemists. One of the aims we have in mind is to introduce a new catalyst for synthesis of 2,4,5-trisubstituted imidazoles with cost effectiveness and mild condition in high yields.
In previous years the Ammonium chloride (NH4Cl) was used as a catalyst for synthesis of organic compounds. For example, the Ammonium chloride was applied in the synthesis of pyrolo [3,4b] pyridines [8], the Claisen rearrangements of aliphatic compounds [9], the reduction of azo compounds to corresponding hydrazines [10] and the reduction of nitro phenols [11]. In 2009, Dr Maleki’s research group became successful to synthesize 2-aryl benzothiazoles by using Ammonium chloride as a catalyst [12]. In this work, we report the solvent-free synthesis of 2,4,5-trisubstituted imidazoles using Ammonium chloride (NH4Cl) as a catalyst under classical heating (Figure 1 ).
We examined a wide variety of aromatic aldehydes with various substituents to establish the catalytic importance of NH4Cl for this reaction. A wide range of ortho-, meta– and para-substituted aromatic aldehydes undergo this one-pot multicomponent synthesis with Benzil or Benzoin and ammonium acetate to afford 2,4,5-trisubstituted imidazoles in good yields.
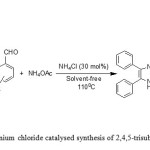 |
Figure 1: Ammonium chloride catalysed synthesis of 2,4,5-trisubstituted imidazoles. |
Experimental
Melting points were measured with an Electrothermal 9100 apparatus. IR spectra were recorded with a Varian 3100 FTIR spectrometer. CHN analyses were performed on Exeter Analytical Inc. ‘Model CE-400 CHN Analyzer’. 1H and 13C NMR spectra were recorded with a BRUKER DRX-400 AVANCE spectrometer at 298 oK and 75.47 MHz, respectively. NMR spectra were obtained on solutions in DMSO-d6. All the products are known compounds, which were characterized by IR and 1H NMR spectral data and their m.p.’s compared with literature reports.
General Procedure For Preparation Of 2a–L
A mixture of aldehyde (1 mmol), benzil or benzoin (1 mmol), ammonium acetate (5 mmol) and ammonium chloride (3 mmol, 30 mol %) as a catalyst in a 20 ml glass tube was stirred at 110 oC for 45-75 min. After completion of the reaction, the reaction was cooled to room temperature and solid materials washed with water and the solvent was evaporated to give the crude product. For further purification it was recrystallized from ethanol 96% to afford pure product.
Selected Spectral Data
2,4,5-Triphenyl-1H-imidazole (2a).
Mp 273–275 oC. FTIR (KBr,cm-1): 3451, 2856, 1636, 1490; 1H NMR (400 MHz, DMSO-d6): δ 12.69 (s, 1H), 8.09 (d, 2H), 7.56–7.22 (m, 13H); 13C NMR (75 MHz, DMSO-d6): δ 145.6, 137.2, 135.2, 131.2, 130.4, 128.7, 128.5, 128.3, 128.2, 127.8, 127.2, 126.6, 125.3; Anal. Calcd for C21H16N2: C, 85.11; H, 5.44; N, 9.45. Found: C, 85.18; H, 5.49; N, 9.33.
2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (2b).
Mp 263 oC. FTIR (KBr, cm-1): 3452, 3065, 1635, 1323; 1H NMR (400 MHz, DMSO-d6): δ 12.78 (s, 1H), 8.11 (d, 2H), 7.56–7.23 (m, 12H); 13C NMR (75 MHz, DMSO-d6): δ 146.3, 130.3, 129.9, 129.2, 128.5, 127.4, 127.0, 126.4, 125.5, 125.2, 123.3, 116.3; Anal. Calcd for C21H15N2Cl: C, 76.24; H, 4.57; N, 8.47. Found: C, 76.20; H, 4.61; N, 8.41.
4,5-Diphenyl-2-p-tolyl-1H-imidazole (2c).
Mp 231–233 oC. FTIR (KBr, cm-1): 3449, 3034, 1611, 1495, 1320; 1H NMR (400 MHz, DMSO-d6): δ 12.59 (s, 1H), 7.98 (d, 2H), 7.54–2.21 (m, 12H), 2.35 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 145.6, 137.6, 136.9, 135.2, 131.1, 129.2, 128.6, 128.3, 128.1, 127.9, 127.6, 127.0, 126.4, 125.1, 20.8; Anal. Calcd for C22H18N2: C, 85.13; H, 5.85; N, 9.03. Found: C, 85.23; H, 5.79; N, 8.97.
2-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (2d).
Mp 229–233 oC. FTIR (KBr, cm-1 ): 3425, 3029, 2956, 1610, 1495, 1249; 1HNMR (400 MHz, DMSO-d6): δ 12.50 (s, 1H), 8.03 (d, 2H), 7.50–7.33 (m, 10H), 7.05 (d, 2H), 3.82 (s, 3H); 13C NMR (75MHz, DMSO-d6): δ 159.5, 145.7, 128.4, 127.7, 126.8, 123.1, 114.1, 55.2; Anal. Calcd for C22H18N2O: C, 80.96; H, 5.56; N, 8.58. Found: C, 80.90; H, 5.51; N, 8.63.
4-(4,5-Diphenyl-1H-imidazol-2-yl)-phenol (2e).
Mp 265-269 oC. FTIR (KBr, cm-1): 3590, 3454, 3284, 3064, 1701, 1283; 1H NMR (400 MHz, DMSO-d6): δ 12.40 (s, 1H), 9.70 (s, 1H), 7.90 (d, 2H), 7.54–7.21 (m, 10H), 6.86 (d, 2H); 13C NMR (75 MHz, DMSO-d6): δ 157.7, 146.1, 136.6, 135.4, 131.3, 128.6, 128.3, 127.5, 127.3, 127.0, 126.8, 126.3, 121.6, 115.4; Anal. Calcd for C21H16N2O: C, 80.75; H, 5.16; N, 8.97. Found: C, 80.79; H, 5.22; N, 8.91.
2-(4-Fluorophenyl)-4,5-diphenyl-1H-imidazole (2f.)
Mp 248-251 oC. FTIR (KBr, cm-1): 3316, 2993, 2470, 1660, 1210, 1169, 874, 719, 639; 1H NMR (400 MHz, DMSO-d6): δ 12.82 (s, 1H), 8.28 (d, 2H), 7.22-7.55 (m, 10H), 7.03 (d, 2H); 13C NMR (75 MHz, DMSO-d6): δ 165.4, 137.3, 131.1, 129.8, 128.9, 127.7, 127.2, 126.6, 125.9, 125.5, 124.1, 117.4; Anal. Calcd for C21H15N2F: C, 76.27; H, 4.54; N, 8.41. Found: C, 76.22; H, 4.60; N, 8.43.
2-(2-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (2g).
Mp 210-213 oC. FTIR (KBr, cm1): 3437, 3033, 2950, 1615, 1498; 1H NMR (400 MHz, DMSO-d6): δ 11.82, (s, 1H), 8.02 (d, 1H), 7.53–7.07 (m, 13H), 3.92 (s, 3H); 13C NMR (75MHz, DMSO-d6): δ 158.2, 146.2, 128.3, 127.5, 125.4, 123.8, 115.4, 55.3; Anal. Calcd for C22H18N2O: C, 80.96; H, 5.56; N, 8.58. Found: C, 80.90; H, 5.63; N, 8.51.
2-(3-Nitrophenyl)-4,5-diphenyl-1H-imidazole (2i).
Mp 299-301 oC. FTIR (KBr, cm-1): 3448, 3068, 1526, 1350; 1H NMR (400 MHz, DMSO-d6): δ 13.10 (s, 1H), 8.95 (s, 1H), 8.53 (d, 1H), 8.23 (d, 1H), 7.81 (d, 1H), 7.54–7.33 (m, 10H); 13C NMR (75 MHz, DMSO-d6): δ 148.4, 143.4, 131.8, 131.2, 130.4, 128.7, 128.4, 127.1, 122.6, 119.4; Anal. Calcd for C21H15N3O2: C, 73.89; H, 4.43; N, 12.31. Found: C, 73.84; H, 4.47; N, 12.39.
Results and Discussions
Several methods are used in the synthesis of these trisubstituted imidazoles and their derivatives.In addition, the synthesis of these heterocycles has been usually carried out in polar organic solvents such as ethanol, methanol, acetic acid, DMF and DMSO leading to complex isolation and recovery procedures. These processes also generate waste containing catalyst and solvent, which have to be recovered, treated and disposed of. The toxicity and volatile nature of many organic solvents, particularly chlorinated hydrocarbons that are widely used in huge amounts for organic reactions have posed a serious threat to the environment [13]. Thus, design of solvent-free catalytic reaction has received tremendous attention in recent times in the area of green synthesis [14].
Efficiency of this reaction is mainly affected by the amount of catalyst, temperature and reaction time. For getting the best conditions, initially we started the condensation of benzil (1 mmol),4-chloro benzaldehyde (1 mmol) and ammonium acetate (5 mmol) in the presence of ammonium chloride (1 mmol, 10 mol %) as a catalyst at 100 oC for 1h, which led to low yield (50%) of 2,4,5-trisubstituted imidazole. To enhance the yield of the desired product the temperature of thereaction was increased to 130 oC. With increasing the temperature, the productivity of the reaction increased but was not very high, yet. Then, it was thought worthwhile to carry out the reaction in the presence of higher amount of the catalyst. As indicated in Table 1, Maximum yield was obtained (91%) when the reaction was loaded with 30 mol % of the catalyst at the 110 oC. A further increasing of catalyst loading does not affect the yield (entry 11, Table 1).
Table 1: Optimization one-pot synthesis of trisubstituted imidazoles under classical heating conditionsa
|
Yield |
Time(min) |
T( |
entry NH4Cl (mol%) |
|
50 |
60 |
100 |
1 10 |
|
56 |
60 |
110 |
2 10 |
|
60 |
60 |
120 |
3 10 |
|
67 |
60 |
130 |
4 10 |
|
70 |
60 |
110 |
5 20 |
|
76 |
60 |
120 |
6 20 |
|
80 |
60 |
130 |
7 20 |
|
91 |
60 |
110 |
8 30 |
|
85 |
60 |
120 |
9 30 |
|
71 |
60 |
130 |
10 30 |
|
90 |
60 |
110 |
11 40 |
a Benzil (1 mmol), 4-chloro benzaldehyde (1 mmol) and ammonium acetate (5 mmol)
After optimizing the conditions, we applied this catalyst for synthesis of trisubstituted imidazoles by using different aromatic aldehydes with a wide range of ortho-, meta– and para-substitutions under solvent-free classical heating conditions to establish the catalytic importance of NH4Cl for this reaction.
Generally, the synthetic procedure involves stirring the mixture of aldehyde (1 mmol), benzil (1 mmol), ammonium acetate (5 mmol) and ammonium chloride (30 mol %) for 45-60 min at 110 oC. The corresponding results are given in Table 2. We found that the reaction proceeded very efficiently either electron-releasing or electron-withdrawing substituents on aryl ring of aldehyde.
Table 2: Synthesis of 2,4,5-triaryl-1H-imidazoles (2a-l) using (30 mol%) ammonium chloride under solvent-free conditions
|
Productsa |
R |
Time (min) |
Yields (%)b |
Mp/ ºC |
|||
|
Benzil |
Benzoin |
Benzil |
Benzoin |
Found |
Reported |
||
|
2a |
4-H |
50 |
60 |
86 |
83 |
273-275 |
272-274 [15] |
|
2b |
4-Cl |
45 |
60 |
91 |
85 |
264-266 |
262-264 [15] |
|
2c |
4-CH3 |
50 |
65 |
88 |
80 |
231-232 |
230-232 [16] |
|
2d |
4-OMe |
55 |
75 |
78 |
75 |
230-233 |
228-231 [15] |
|
2e |
4-OH |
45 |
55 |
85 |
77 |
265-267 |
268-270 [17] |
|
2f |
4-F |
50 |
60 |
86 |
80 |
250-252 |
250-251 [18] |
|
2g |
2-OMe |
60 |
65 |
85 |
81 |
212-213 |
210-211 [17] |
|
2h |
3-Br |
45 |
50 |
90 |
85 |
232-233 |
231-233 [19] |
|
2i |
3-NO2 |
60 |
70 |
85 |
80 |
301-302 |
>300 [19] |
|
2j |
2-CH3 |
55 |
60 |
90 |
80 |
201-204 |
205-207 [20] |
|
2k |
4-Br |
45 |
65 |
88 |
75 |
264-266 |
263-265 [16] |
|
2l |
3,4-diOMe |
55 |
60 |
85 |
80 |
217-219 |
216-218 [21] |
aAll the isolated products were characterized on the basis of their physical properties and IR, 1H-and 13C-NMR spectral analysis and by direct comparison with authentic materials; bIsolated yields
Also due to direct use of benzoin rather than benzil in the synthesis of imidazoles a significant improvement in the synthesis toward the greener chemistry is represented. We have repeated the reaction with benzoin instead of benzil and the desired product has been efficiently produced.
As indicated in Table 2, when we used benzoin instead of benzyl, the reaction time increased and also the yield of the reaction decreased partially.
Possible mechanism for the NH4Cl catalysed synthesis of trisubstituted imidazoles has been proposed in Figure 2. In summary, this paper describes a convenient and efficient process for the Solvent-free synthesis of trisubstituted imidazoles through the three-components coupling of benzil or benzoin, aldehydes and ammonium acetate using NH4Cl as a catalyst. Reaction profile is very clean and no side products are formed. All the synthesized imidazoles have been characterized on the basis of elemental and spectral studies. We believe that this procedure is convenient, economic, and a user-friendly process for the synthesis of trisubstituted imidazoles of biological and medicinal importance.
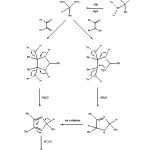 |
Figure 2: Probable mechanism for the formation of triarylimidazoles using benzil or benzoin, ammonium acetate, aromatic aldehydes and ammonium chloride as catalyst. Click here to View figure |
Conclusion
We have been able to introduce an efficient and environmentally friendly approach for the synthesis of biologically active trisubstituted imidazoles via condensation of benzil or benzoin with various aromatic aldehydes and ammonium acetate using ammonium chloride as a catalyst.High yields, easy work-up and purification of compounds by non-chromatographic method (crystallization only) are the key advantages of this method.
References
- D’Souza D. M. and Mueller T. J. J., Chem. Soc. Rev., 36, 1095 (2007).
- Domling A., Chem. Rev., 106, 17 (2006).
- Tempest P. A., Curr. Opin. Drug. Discov. Devel., 8, 776 (2005)
- Kalinski C., Lemoine H., Schmidt J., Burdack C., Kolb J., Umkehrer M. and Ross G., Synlett., 24, 4007 (2008).
- Gadekar L. S., Mane S. R., Katkar S. S., Arbad B. R. and Lande M. K., Cent. Eur. J. Chem., 7, 550 (2009).
- Radziszewski B., Chem. Ber., 15, 1493 (1882).
- Japp F. and Robinson H., Chem. Ber., 15, 1268 (1882).
- Janvier P., Sun X. and Bienayme H., J. Am. Chem. Soc., 124, 2560 (2002).
- Ralls J. W., Lundin R. E. and Bailley G. F., J. Org. Chem., 28, 3521 1963).
- Sridhara M. B., Srinivasa G. R. and Gowada D. C., J. Chem. Res. S., 1, 74 (2004).
- Basu M. K., Becker F. F. and Banik B. K., Tetrahedron Lett., 41, 5603 (2000).
- Maleki B., Azarifar D., vaghei R. G., Veisi H., Hojati S. F., Gholizadeh M., Salehabadi H. and Moghadam M. K., Monatsh Chem., 140, 1485 (2009).
- Nelson W. M., Anastas P. T. and Williamson T. C., Green Chemistry, Oxford University Press, Oxford, 200 (1998).
- Tanaka K. and Toda F., Chem. Rev., 100, 1025 (2000).
- Kidwai M., Mothsra P., Bansal V. and Goyal R., Montsh Chem., 137, 1189 (2006).
- Safari J., Dehgan Khalili S. and Banitaba S. H., J. Chem. Sci., 122, 437 (2010).
- Sangshetti J. N., Shinde D. B., Kokare N. D. and Kotharkar S. A., Montsh Chem., 139, 125 (2008).
- Yu C., Lei M., Su W. and Xie Y., Synth. Commun., 37, 3301 (2007).
- Samai S., Nandi G. C., Singh P. and Singh M. S., Tetrahedron, 65, 10155 (2009).
- White D. M. and Sonnenberg J., J. Org. Chem., 29, 1926 (1964).
- Zhou J. F., Song Y. Z. and Tu S. J., Synth. Commun., 35, 1369 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









