Adsorption Isotherms Of Diethyl And Dimethyl Malonate Esters On Single –Walled Carbon Nanotubes
Mehdi Vadi and Azim Lorestani
Department of Chemistry, Marvdasht Branch, Islamic Azad University, Marvdasht, Fars, Iran.
The purpose of this research was to study the adsorption behavior of diethyl and dimethyl malonate esters on the single-walled carbon nanotubes (SWCNTs), by Uv-Vis lambda 45 spectrophotometer. In this work we used five concentrations of esters (100-150-200-250-300 µgml-1). In all experiments the condition such as adsorbate, contact time, temperature (295 0 K) and pH of sample were constant. The experimental results obtained at the temperature 2950K with equilibrated adsorption were studied and were represented by the Langmuir, Freundlich and Temkin isotherm models. The experimental data showed that Freundlich isotherm has the best compatibility.
KEYWORDS:Adsorption; Diethyl malonate ester; Dimethyl malonate ester; Carbon nanotubes
Download this article as:| Copy the following to cite this article: Vadi M, Lorestani A. Adsorption Isotherms Of Diethyl And Dimethyl Malonate Esters On Single –Walled Carbon Nanotubes. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Vadi M, Lorestani A. Adsorption Isotherms Of Diethyl And Dimethyl Malonate Esters On Single –Walled Carbon Nanotubes. Available from: http://www.orientjchem.org/?p=22931 |
Introduction
Before the discovery of carbon nanotube (CNT) which had new shape of carbon-carbon bond [1], two types of carbon-carbon bonds was known in nature like graphite and diamond.
Graphite consists of layers of carbon atoms, which form distinct units of carbon atoms in the six vertices of the hexagonal arrangement are located. Diameter of nanotubes is between 1 to 2 nanometers to several microns in length. At the end of each nanotubes may be blocked by half of a fullerene, therefore the end of it may be connected to hexagon or pentagon [2]. But the most important factor in determining the properties of the nanotubes is known as torsion [2.3.4.5]. The other structural factor of nanotubes is layer forms which can be multilayer and monolayer. Each of these forms has several applications. Crystalline structure of carbon nanotubes has pore structure with high effective area and this characteristic cause relatively good adsorption of polymers. The main advantage of carbon nanotubes are conduction properties. Both of conductive and nonconductive carbon nanotubes are used in electrical applications . All of the compounds on the surface of CNTs are absorbed two main covalent bonds and non covalent bonds[ 6.7]. Absorption takes places on the surface and on the carbon walls while non covalent absorption which is the kind of physically absorption takes place on the CNTs walls. One of the characteristics of non covalent bonds adsorption on CNTs is it that, the structure of CNTs, doesn’t change after this absorption and separating the absorption .Carbon nanotubes (CNTs) these compounds highly developed in chemical and physical demention. The ways of synthesis of this compound developed quickly [8]. CNTs can adsorb so many of atoms and molecules on their surface such as adsorption of metallic elements like Lithium [9] , Potassium [10] , Rubidium [11] , cesium [12] , and non metallic such as Hydrogen [13] , Oxygen [14] , Nitrogen [15], and Methanol[16] . Because of high energy level of these compositions, low diameter nanotubes have high tension strength about 100 GPa [3.5]. Other properties of nanotubes are van der waals force between atoms and therefore lead to have very low ability to bind to each other (the unique electrical properties) in the metallic and semiconducting nanotubes [3.5.2.17], the conductivity in the longitudinal direction [3.2], thermal conductivity and field emission properties [3.18.19].
The field emission properties of structures with length to diameter ratio (greater than 1000) and sharp vertex in atomic structure, high thermal and chemical stability, and high thermal and electrical conductivity can be seen [19.20]. The single-layer carbon nanotubes have nanoscale channels with a surface area of 400 square meters. This characteristic provides the ability to adsorb gases [21].
Esters are groups of organic compounds that are derivatives of carboxylic acids in which the carboxyl group is connected to alkyl group instead of hydrogen. Malone diethyl ester, also known as DEA, which is one type of acid diethyl ester Malonic acid can be find naturally in grapes, colorless liquid with an odor similar apple. DEA also is used in perfumes and synthesis compounds such as barbiturates, add artificial and vitamin B1 and vitamin B6 [22.23].
Dimethyl Malonate is diester derivative of Malonic acid that these compounds are used for organic synthesis [24].The purpose of this work is to study of adsorption behavior of diethyl and dimethyl malonate Esters on single walled carbon nanotubes as function of adsorbtion isotherms. Molecular structures of this ester are shown in figure 1.
Dimethyl malonate C5H8O4 (1)
Diethyl malonate C7H12O4 (2)
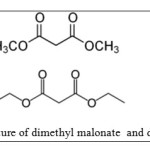 |
Figure 1 The structure of dimethyl malonate and diethyl malonate |
Materials and Methodes
Dimethyl and diethyl malonate esters with purity of 78% and 80% respectively were purchased from Merck. Single walled carbon nanotubes as adsorbant with purity of 95% were purchased from Aldrich. The Uv-Vis spectra were performed by using Lambda 45 Uv-Vis spectrophotometer.
Adsorption experiments
A stock solution of about 1000 mg L-1 diethyl malonate and dimethyl malonate were prepared. The range of malonate ester concentration used is 100 to 300 mg L-1 . After preparing of the solutions, the adsorption of them is monitored, by using spectrophotometer and drawing calibrated curve afterward. Then we were added 0.01 g single walled carbon nanotube to 10 ml standard solution. The mixtures were shaken for 60 min and then the equilibrium adsorption experiments were conducted.
Results and Disscution
Adsorption isotherms
The adsorption isotherm described the relationship between the amount of a substance adsorbed and its remaining amount in the solution in equilibrium and was the method used to evaluate the mechanism of adsorption [29].
Modeling of the adsorption isotherms
Langmuir model
This model of adsorption is used more than the other methods and is expressed by equation (1) [25].
In this equation, qe (mg.g-1) is amount of absorbed material in absorbent surface and qm is equilibrium constant of adsorption and b is the capacity of adsorption in saturated single layer and Ce (mg.L-1) is solution in equilibrium state.
Freundlich model
This model is based on experimental equation which is used more for comprehension of metallic ions adsorption on the heterogeneous surface with multi layer adsorption and also for adsorptions that can be increased unlimited with increasing intension [26]. This model is specified with equation (2).
In this equation, qe (mg.g-1) denotes the amount of absorbed material in absorbent surface, K and n are adsorption capacity and adsorption intensity respectively.
Temkin model
Temkin isotherm represents clearly the reaction between absorbent and absorbed particles [27.28] . This model is specified with equation (3).
In this relation, A is equivalent of bond constant with maximum of connect energy, b is constant of Temkin isotherm and B is relevant to adsorption temperature.
Isotherms of the adsorption process of esters on carbon nanotubes are shown in figures 2 to 4 and parameters of these models are calculated and shown in table1, the amounts of adsorption for different concentration are shown in table 2.
The isotherms of esters adsorption trend on the carbon nanotubes are shown in the Fiqure 2 and 4. All the parameters of this method are calculated and shown in Table 1 and the amount of adsorption in various concentrations is shown in table 2.
Table 1: Parameters and correlation coefficient of adsorption isotherms models
| Langmuir model | Freundlich model | Temkin model | ||||||||
| b | q | R2 | n | Kf | R2 | A | B | b | R2 | |
| Diethyl malonate | 0.0132 | 36.0974 | 0.993 | 3.1473 | 4.8035 | 0.9934 | 0.5825 | 7.5405 | 3.208 | 0.9838 |
| Dimethyl malonate | 0.0102 | 36.1314 | 0.9917 | 2.6637 | 3.2754 | 0.9976 | 0.6522 | 8.1502 | 2.9680 | 0.9881 |
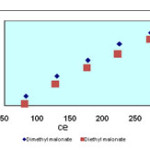 |
Figure2. Langmuir isotherm of malonate esters on SWCNs |
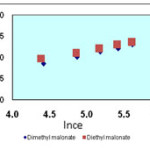 |
Figure 3. Freundlich isotherm of malonate esters on SWCNTs |
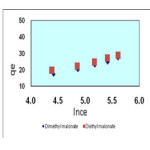 |
Figure 4. Temkin isotherm of malonate esters on SWCNTs Click here to View figure |
Table2. The amounts of esters on carbon nanotube
| Adsorption | Adsorption | |
| Ci(mg/g) |
Diethyl malonate ester ( mg/L) |
Dimethyl malonate ester mg/L)) |
| 100 | 0.0742 | 0.0874 |
| 150 | 0.1147 | 0.1330 |
| 200 | 0.1552 | 0.1788 |
| 250 | 0.1957 | 0.2254 |
| 300 | 0.2370 | 0.2715 |
Conclusion
In this research, the adsorption of esters (dimethyl malonate and ethyl malonate ester ) on the carbon nanotubes were studied. As is shown in Table 2.with increasing ester concentration.the adsorption on carbon nanotubes is increased. Considering the similar structures and because of electron donating conductive effect of ethyl groups( with respect to methyl), adsorption of diethyl malonate with respect to dimethyl malonate ester is higher. Therefore diethiy malonate can interact with p center of carbon nanotubes much eastier. In fact this electronic richness is a kind of booster for interaction of carbonyl group with double bound of carbon nanotube. On the other hand. Data related to equilibrium condition have been properly illustrated by Langmuir, Freundlich and Temkin. The constants of these models confiem the experimental observation and are given in Table.
References
- Iijima S.,Nature 354(1991)56.
- Mildred D. , Gene D. , Peter E. , Richiro S., Carbon nanotubes. Physics World 1998; Issue ]Nanotechnology Opportunity Report II
- [Thomas A. A. Physical properties of carbon nanotubes. Science, Engineering and Technology 2000
- [Hongjie D. , Tom G. . AN INTRODUCTION TO CARBON NANOTUBES. Polymer Interfaces and Macromolecular Assemblies 2003
- Bahr, J.L.; Tour, J.MJ.Mater.Chem.2002, 12, 1952
- Basiuk,E.V.;Monroy-Pelaez,M,Puente-Lee,I;Basiuk,V.A.Nano Lett,2004,4,863
- Chen,R.J;Zhang,Y;Wang;D;Dai,H,J.Am.Chem.Soc.2001,123,3838
- N.Bendiab, E.Anglaret, j-l.bantignies, A.Zahab, J.L.Sauvajol, P.Petit, C.Mathis and S.Lefrant, Phy, Rev.B64 (2001), P.245424
- A.S.Claye, N.M.Nemes, A.Janossy and J.E.Fischer, Phy. Rev. B62 (2002), P. R4845
- A.M.Rao, P.C.Eklund, S.Bandow, A.Thess and R.E.Smalley, Nature 3881 (1997), P.257
- A.Wadhawan, R.E.Stallcup II and J.M.Perez, Appl. Phys. Lett.78 (2001), P.108
- A.Cao, H, Zhu, X.Zhang, X.Li, D.Ruan, C.Xu, B.Wer, J.Liang and D.Wu.Chem. Phy.Lett.342 (2001), P.510
- X.Y.Zhu, S.M.Lee andT.Frauenheim, Phys. Rev. Lett. 85(2000), P.2757
- Q-H.Yang, P-X.Hou, S.Bai, M-Z.Wang and H-M. Cheng, Chem. Phys, Lett. 345 (2001), P.18
- S.Talapatra and A.D.Migone, Phys. Rev. B56 (2002), P.045416
- Sigma Aldrich. Fullerenes and Carbon Nanotubes – Structure, Properties and Potential Applications.
- Philip G. Collins and A. Zettl, Unique characteristics of cold cathode carbon-nanotube-matrix. field emitters. Phys. Rev. 1997; 9391 – 9399
- Saito R. , Dresselhaus G. , Dresselhaus M. S. IEEE Electrical Insulation Magazine 1998
- Julian H. G. , Milo M. S. , Molly M. S. Nanofibrous Materials for Tissue Engineering. Journal of Experimental Nanoscience 2006; 1 (1) p 1
- Massoud Rostan – Abadi, Sandeep Angihotri, Mark, J. Rood, “Energy and Enironmental Applications of Carbon Nanotubes “, Proceeding of the First Conference on Nanotechnology The Next Industrial Revolution, Volume 1,2002,Tehran
- IR spectrum of Malonic acid.
- C. S. Palmer and P. W. McWherter, “Ethyl Bromoacetate”, Org. Synth., ; Coll. Vol. 1: 24
- Merck Index, 11th Edition, 6009.
- F. Martinez, A. Hernandez, ‖Protein adsorption onto microfiltration membranes: The role of solute-solid.
- Jeong-yeol yoon,woo-sik kim,‖Adsoption of BSA on highly carboxylate microspheres quantitative effects of surface functional groups and interaction forces‖,J.Colloid and Interface Sci.,177 ، 613-620 ، 1996
- M. Özacar, İ.A. Şengil, Bioresour. Tecnol., 96 (2005) 791-795.
- I.D. mall, V.C. Srivastava, N.K. Agarwall, I.M. Mishra. ChemospHere, 61(2005) 492-501
- N.M.p.Dias,L.R.F.Alleoni,J.c.casagrande and o.A.camargo,Rev.Bras.Eng.Agric,Amb,5,229, 2001.

This work is licensed under a Creative Commons Attribution 4.0 International License.









