A Review: Synthesis, Characterization and Cell Performance of Cu2O Based Material for Solar Cells
Hafsa Siddiqui, Mohammad Ramzan Parra, Padmini Pandey, Neha Singh, M. S. Qureshi and Fozia Z. Haque*
Department of Physics, Maulana Azad National Institute of Technology, Bhopal, India.
Low-cost thin film oxide/oxide heterojunctionsbased photovoltaic solar cellsare one of the alternatives to silicon solar cells, among the potential photovoltaic devices based on semiconductor oxides.Cuprous oxide is a potential material for the fabrication of low cost solar cells for terrestrial application. In this article, firstly, we reviewed cuprous oxide Crystal structure, Band structure, different properties of cuprous oxide material such as electrical and transport properties andphotoluminescence.Then we discuss in detail the synthesis techniques for the production of copper oxide such as Thermal Oxidation, Anodic Oxidation, Electrodeposition, Sputtering, Chemical vapor deposition etc. Latter on a detailed survey on the previous work so far carried out on Cu2O based solar cells is presented.Thefabrication and cellperformance of based Solar Cells is also discussed.
KEYWORDS:Cell performance; Solar Cells; Low-cost thin
Download this article as:| Copy the following to cite this article: Siddiqui H, Parra M. R, Pandey P, Singh N, Qureshi M. S, Haque F. Z. A Review: Synthesis, Characterization and Cell Performance of Cu2o Based Material for Solar Cells. Orient J Chem 2012;28(3). |
| Copy the following to cite this URL: Siddiqui H, Parra M. R, Pandey P, Singh N, Qureshi M. S, Haque F. Z. A Review: Synthesis, Characterization and Cell Performance of Cu2o Based Material for Solar Cells. Orient J Chem 2012;28(3). Available from: http://www.orientjchem.org/?p=23212 |
Introduction
The need for sustainable power generation has encouraged research into a variety of photovoltaic materials and structures, with a greater emphasis being placed on a balance between performance and cost. The stability of many semiconducting oxides relative to other inexpensive solar cell technologies, such as organic [1] and dye-sensitized [2] cells, makes them an attractive alternative. Yet low-cost, non-toxic, inorganic solar cell technologies have received comparatively little attention. In a recent report, nine inorganic semiconductors were identified as having both the potential for annual electricity production in excess of worldwide demand and material extraction costs less than that of crystalline silicon [3].Further to materials costs, a recent study examined the high cost of modern vacuum deposition methods and highlighted the need for low-temperature, atmospheric, solution-based synthesis [4–7].Copperhas two stable oxides: cupric oxide (CuO) and cuprous oxide (Cu₂O). These two oxides are semiconductors with band gaps in the visible or near infrared regions [8]. These materials have several advantages: (i) availability and abundance of the starting materials (ii) non-toxic nature (iii) Low production cost (iv) Band gaps lie in an acceptable range for solar energy conversion, and (v) n- and p-type conductivity [9-11].Copper and copper oxide (metal-semiconductor) are one of the first photovoltaic cells invented [12]. Its semiconductor properties and the emergence of photovoltaic effect were discovered by Edmond Becquerel in 1839 while experimenting in the laboratory of his father, Antoine-César Becquerel. Copper (I) Oxide (Cu₂O), in particular, has been synthesized extensively in polycrystalline form by electrodeposition from solutions near room temperature [5, 13, 14]. Cu₂O has a direct band-gap of 2.17 eV which is suitable for photovoltaic conversion. Theoretical calculations have predicted an electrical power conversion efficiency of approximately 9-12 %.The practical electrical power conversion efficiencies obtained by researchers, in the past, are below 2% [15]. However, recently efficiency of 3% has been reported [16].The researches carried out during the mid-seventies and early eighties have now helped in revealing some of the mysteries surrounding this material and a perfect understanding of the various causes for the poor performance of Cu₂Osolar cells are now known.
In this review the developments of Cuprous Oxide (Cu₂O) solar cells are reviewed. We discuss the properties of Cuprous Oxide and the methods of the production of Cuprous Oxide. Subsequently, a discussion on the performance of these cells is reported. Finally, the conclusion and outlook to further improve Cuprous Oxide solar cell fabrication and performance are presented.
Crystal structure of Cu₂O
The unit cell of Cu₂O with a lattice constant of 0.427 nm is composed of a body centered cubic lattice of oxygen ions, in which each oxygen ion occupies the center of a tetrahedron formed by copper ions. The Cu atoms arrange in an fcc sublattice, the O atoms in a bcc sublattice. The unit cell contains 4 Cu atoms and 2 O atoms. One sublattice is shifted by a quarter of the body diagonal. The space group is Pn3m, which includes the point group with full octahedral symmetry. This means particularly that parity is a good quantum number. Figure 1 shows the crystal lattice of Cu₂O. Cuprous oxide (copper (I) oxide Cu₂O) is found in nature as cuprite and formed on copper by heat. It is a red color crystal used as a pigment and fungicide [17].
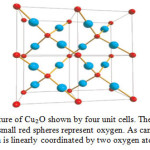 |
Figure 1: Crystal structure of Cu₂O shown by four unit cells. The big turquoise spheres represent copper; the small red spheres represent oxygen. As can be seen, each copper atom is linearly coordinated by two oxygen atoms. |
Band structure of Cu₂O
Cuprous oxide (Cu₂O) is a direct gap semiconductor with band gap 2.17 eV[11-19]. Cu₂O crystals have a simple cubic lattice with two formulas unit per unit cell and have the symmetry of space group . There are two way of choosing the unit cell. In one, the oxygen atoms form a bcc lattice while in other, the copper atoms form fcclattice. The site symmetries of the oxygen and the copper are Td and T 3d respectively. Several band structure calculations have followed the initial Augmented–Plan–Wave (APW) calculation by Dahl and Switendick [20].Band structure calculations show that the lowest energy band-to-band transition is Direct at the Γ pointof the Brillouin zone as deposited in figure 2. The four transitions are named according to the wavelengths of their spectral positions yellow, green, blue, and indigo, respectively (see Figure 2).At the zone center the valence band is largely comprised of copper 3d orbitals and has Γ7+ symmetry, while the conduction band is made up of copper 4s orbitals[21] and has Γ6+ .An optical transition between these band would be s-˃ d in an atomic sense, making it parity forbidden (Γ7+→Γ6+ , Γ8+→ Γ6+).On the left of Figure 2, the band dispersion is shown schematically. It has been studied by cyclotron resonance experiments [22-34].
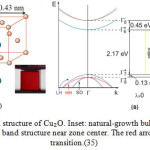 |
Figure 2: (a) Crystal structure of Cu₂O. Inset: natural-growth bulk Cu₂O crystal. (b) Schematic of the Cu₂O band structure near zone center. The red arrow denotes quadrupole transition.(35) |
Properties of Cuprous Oxide
Electrical and transport properties of Cuprous Oxide
as a result of stoichiometry, is the major active impurity and gives a p-doped semiconductorCopper oxide is a defect semi-conductor but nowadays it is well established that Cu₂O is a natural p-type semiconductor, whose carrier concentration depends on the amount of cation deficiency [36], cuprous oxide is one of such non-stoichiometric materials with formula Cu2-δO. The deviation from stoichiometry, δ is generally attributed to some imperfections. Sears et al. 1984 [37] reported that an excess of oxygen.
![Figure 3: Formation energies for intrinsic p-type defects in Cu₂O in (a) Cu-rich–O-poor conditions and (b) Cu-poor–O-rich conditions. The solid dots denote the transition levels.[3]](http://www.orientjchem.org/wp-content/uploads/2012/09/Vol28No4_Rev_Haf_fig31-150x150.jpg) |
Figure 3: Formation energies for intrinsic p-type defects in Cu₂O in (a) Cu-rich–O-poor conditions and (b) Cu-poor–O-rich conditions. The solid dots denote the transition levels.[3] |
Photoluminescence property of copper oxide
All copper oxides have one thing in common that they are weak luminescent or even nonluminescent systems. In particular, is ‘‘dark’’ black and luminescence data on this material have rarely been collected and reported[46-50]. Whoever Cu2O unambiguously shows several luminescence signals, however, these are weak in intensity. The latter is due to the fact that optical transitions require parity change, which is not given between the energetic highest valence band and lowest conduction band of the direct semiconductor Cu2O . The corresponding spin forbiddance of the transition resulting in the weak luminescence is partly broken by three possible effects: (a) defects within the crystal, (b) the decay of ortho-excitons, or (c) phonon assisted transitions.The majority of the luminescence studies reported were performed on thermally oxidized copper(Figure 4), and they observed broad luminescence bands of have been assigned to doubly charged oxygen vacancies (V02+) at 1 .72 eV (720nm)singly charged oxygen vacancies (VO2+) at 1.53 eV (810 nm) and copper vacancies (VCu) at This assignment is very consistently used in literature [51-55], but the exact peak positions and especially the relative intensities strongly depend on the used growth process as well as sample processing. The two high-energy bands were only observed at low measuring temperatures in the past, whereas the copper-vacancy band at low energies dominates the spectra at room temperature. In high-quality material, e.g., floating-zone grown single crystals, one can observe the additional recombination of the 1s ortho-exciton via an electric quadruple transition at temperatures of 4.2K at an energy of 2.03 eV (610 nm ), and respective phonon side bands via electric dipole transitions.
![Figure 4: Absorption spectra of Copper oxide. The horizontal and vertical dotted lines indicate the energies where the absorption coefficient reaches 1 x 105 cm-1 in the spectra[3]](http://www.orientjchem.org/wp-content/uploads/2012/09/Vol28No4_Rev_Haf_fig41-150x150.jpg) |
Figure 4: Absorption spectra of Copper oxide. The horizontal |
The optical and electrical properties of absorber materials in solar cells are key parameters which determine the performance of solar cells. Hence, it is necessary to tune these properties properly for high efficient device. Electrical properties of Cu2O, such as carrier mobility, carrier concentration, and resistivity are very dependent on preparation methods. Cuprous oxide thin films have been prepared by various novel techniques for the synthesis of cuprous oxide i.e. reactive sputtering, sol-gel technique, plasma evaporation, thermal oxidation, chemical vapor deposition, anodic oxidation, reactive sputtering, electrodeposition etc. Each of these methods has its own advantages and disadvantages.
Thermal Oxidation
This is by far the most widely used method of producing Cu2O for the fabrication of solar cells. The procedure involves the oxidation of high purity copper at an elevated temperature (1000 -1,500° C) for times ranging from few hours to few minutes depending on the thickness of the starting material (for total oxidation) and the desired thickness of Cu2O (for partial oxidation).The oxidation process can be carried out either in pure oxygen or in laboratory air. Cu2O has been identified to be stable at limited ranges of temperatures and oxygen pressure. It has been indicated that during oxidation, is formed first and after a sufficiently long oxidation time, Cu2O is formed. However, at temperatures below 1000°C and at atmospheric pressure, mixed oxides of and are formed as observed from the X-ray diffraction ) results. It has been suggested that the probable reactions that could account for the presence of in layers oxidized below are [56].
The unwanted CuO can be removed using an etching solution containing Fel3, HCl and . The oxidation process is followed by annealing the sample at 500°C and then stopping the process by quenching in cold water. This process leads to good quality polycrystalline Cu2O with the bulk resistivity in the range of 102-104 ohm cm.[57-58].The resistivity can further be lowered by oxidizing in the presence of chlorine gas. Resistivity’s below 100 ohm cm have been reported in the literature [59-60] using this procedure. It is also worthy of note that the purity of the starting Cu2O material can have a significant impact on the quality of Cu2O and the performance of the resulting solar cell. A number of pre- and post-oxidation treatments have been suggested in the literature [60] which involves cleaning, etching, polishing and annealing the material prior to and after the oxidations.
Anodic oxidation
Anodic oxidation of copper in alkaline solution is one of the standardmethodologies for producing cuprous oxide powders used for marine paints and for plants preservation. Those powders are composed of particles of micrometer scale. However, solar cells, for their part, require particles or films of much smaller dimensions in order to achieve higher efficiency. Passive protecting layers formed on copper during anodic oxidation in alkaline solutions are widely investigated and described in electrochemical literature. The structure of those films formed on copper in neutral and alkaline solutions consists mainly of Cu2O and Cu2O orCu(OH)2or Stankovićet al. (1998; 1999)[61] investigated the effect of different parameters such as temperature pH, and anodic current density on CuO powder preparation.The lowest valueof average crystallite size was obtained at pH 9.62 , whereas the highest value was obtained at .Singh et al. (2008)[62] reported synthesis of nanostructured by anodic oxidation of copper through a simple electrolysis process employing plain water as electrolyte. They found two different types of Cu2O nanostructures. One of them belonged to particles collected from the bottom of the electrolytic cell, while the other type was located on the copper anode itself.
Sputtering
Cathode sputtering is essentially one of the methods used for the preparation of thin films. The method requires very low pressure in the working space and therefore makes use of vacuum technique. The material to be sputtered is used as a cathode in the system in which a glow discharge is established in an inert gas at a pressure of 10-1-10-2torr and a voltage of a few kilovolts. The substance on which the film is to be deposited is placed on the anode of the system. The positive ions of the gas created by the discharge are accelerated towards the cathode (target). Under the bombardment of the ions the material is removed from the cathode (mostly in the form of neutral atoms or in the form of ions). The liberated components condense on surrounding areas and consequently on the substrates placed on the anode. Reactive sputtering is used in the production of Cu2O . A chemical reaction that occurs with the cathode material (Cu in this case) by the active gas (oxygen) either added to the working gas or as the working gas itself. The resistivity of the deposited Cu2O film can be controlled over a wide range by simply varying the oxygen pressure. Cu2O films of resistivity as low as has been reproducibly obtained [63].
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high performance solid materials. The films may be epitaxial, polycrystalline or amorphous depending on the materials and reactor conditions. Chemical vapor deposition has become the major method of film deposition for the semiconductor industry due to its high throughput, high purity, and low cost of operation. Several important factors affect the quality of the film deposited by chemical vapor deposition such as the deposition temperature, the properties of the precursor, the process pressure, the substrate, the carrier gas flow rate and the chamber geometry. Kobayashi et al.(2000)[64] investigated the high-quality Cu2O thin films grown epitaxial on MgO (110) substrate by halide chemical vapor deposition under atmospheric pressure. CuI in a source boat was evaporated at a temperature of , and supplied to the growth zone of the reactor by carrier gas, and was also supplied there by the same carrier gas. Partial pressure of CuI and O2 were adjusted independently to 1.24× 10-2 and 1.25×103Pa. They found that the optical band gap energy of Cu2Ofilm calculated from absorption spectra is 2.38 eV. The reaction of CuI and O2 under atmospheric pressure yields high quality Cu2O films.
Electrodeposition
Electrodeposition is a low‐temperature processing method where films are deposited from solution precursors. The advantages include the elimination of heat treatments which can be especially meaningful for nanostructures, an effective control of the deposition rate via over potential, the possibility to control the shape or crystallographic orientation via the pH or the precursor additives. It is a very low cost method since it does not require any vacuum processing, Cu2O can be electrodeposited between 30-90°C using a mixture of alkaline cupric sulfate, lactic acid and NaOH. Thickness ranges from 0.5-7μm . The potential must be properly controlled to allow electrodeposition of Cu2O as opposed to elemental Cu. Deposited films can be annealed. The reaction is
This technique typically yields a polycrystalline structure with the average grain size between . Electrochemical deposition technique is a simple, versatile and convenient method for producing large area devices. Low temperature growth and the possibility to control film thickness, morphology and composition by readily adjusting the electrical parameters, as well as the composition of the electrolytic solution, make it more attractive. At present, electrodeposition of binary semiconductors, especially thin films of the family of wide bend gap II-IV semiconductors, from aqueous solutions is employed in the preparation of solar cells. A photovoltaic device composed of a p-type semiconducting cuprous (I) oxide (Cu2O ) and n-type metal oxides has attracted increasing attention as a future thin film solar cell, due to a theoretical conversion efficiency of around 20% and an absorption coefficient higher than that of a Si single crystal [65].The parametersof the solar cells, such the open circuit voltage (VOC ), the short circuit current (ISC), the fill factor(FF) , the diode quality factor(n) , serial (RS) and shunt resistant(RSh) and efficiency(η )were determined. The barrier height (Vb )can also be determined from capacity-voltage characteristics.
Fabrication and cell performance of Cu2O based solar cells
Fabrication of Cu2O Solar Cells
The experimental and theoretical studies conducted at Joint Centre for Graduate Studies (JCGS) group highlighted more on the potential viability of Cu2O for low cost photovoltaic (PV) power generation. However, in recent times, single polycrystalline Cu2O used for solar cells are obtained via any of the methods presented earlier in this paper. The Schottky barrier solar cells and the heterojunctions have been extensively investigated. Also, in the past, workers have not succeeded in producing n – type Cu2O and therefore a homojunction cell structure (Trivich, 1982) could not be fabricated. Recently,Cu2O homojunction solar cells have been fabricated (Longcheng and Meng, 2007 and Kunhee and Meng, 2009).
Schottky Barrier Solar Cells
As a metal is in contact with a semiconductor, a Schottky barrier is formed. Theoretically, the Schottky barrier height is only determined by the work function of the metal (ΦM)and the electron affinity of the n-type semiconductor . For a p-type semiconductor, the higher the work function of the metal, the easier it is to form an ohmic contact. While for an n-type semiconductor(xs), the lower the work function of the metal, the easier it is to form an ohmic contact.(ΦM=4.7 eV)Copper is used as top metal contact for p-type cuprous oxide, while aluminum (ΦM=4.7 eV) is used as top metal contact for n-type cuprous oxide. A front wall Schottky barrier solar cell (Olsen et al.,1980) is usually fabricated by evaporating a metal on top of Cu2O in high vacuum chambers. A number of metals on Cu2O have been tried and the best performance has been obtained with a natural Cu/Cu2O junction. A new method of obtaining Cu2O has been reported (Iwanoski et al., 1985). This technique uses hydrogen ion bombardment of Cu2O surface thus reducing the top surface to copper. Schottky barrier solar cells can also be fabricated in the back wall structure. This mode requires a natural junction using copper or any other material as the base and depositing a layer of on top of the metal base. The light then illuminates the junction through the semiconductor side. But back wall cells require thin layer of Cu2O(<1μm) because of the high absorption coefficient and low diffusion length of the minority carriers (Trivich et al., 1978). Partial thermal oxidation can be used to produce the thin layer. Back wall solar cells of Cu2O offer greater advantage over front wall cells because of the mechanical stability of the base materials and simplicity of fabrication.
Heterojunction Solar Cells
A heterojunction solar cell is fabricated by depositing n-type semiconductor of suitable band gap on Cu2O.Methods like vacuum deposition, sputtering and electro-deposition have been used for the deposition. Several heterojunction solar cell structures have been studied and reported (Herion et al., 1980). Examples are the zinc oxide- cuprous oxide ( ZnO/Cu2O) and cadmium oxide-cuprous oxide ( CdO/Cu2O) solar cells. The Cu2O/ZnO junction is an ideal abrupt heterojunction at thermal equilibrium and using the known measured values of electron affinities, band gaps and carrier densities in ZnO and Cu2O ,Ea is estimated approximately ˜ 1-1.1 eV (as shown in Figure 5) [66, 67, 68] This value is approximately in the range of extracted activation energies. Thus, it is concluded that the interface recombination is the dominant current flow mechanism across the Cu2O/ZnO heterojunction where the rate limiting step is diffusion of holes against the barrier established by band bending to recombine with electrons trapped in interfacial states. This mechanism is shown as path 1 in Figure 5.
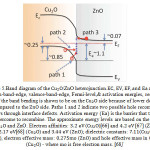 |
Figure 5.Band diagram of the Cu2O/ZnO heterojunction. ,are the conduction-band-edge, valence-band-edge, Fermi-level,& activation energies, respectively. |
Most of the band bending is shown to be on the side because of lower doping in compared to the side.Paths 1 and 2 indicate two possible hole recombination pathways through interface defects.Activation energy is the barrier that the holes have toovercome to recombine.The approximate energy levels are based on the properties of bulk Cu2O and ZnO.Electron affinities:3.2 eV(Cu2O)[66] 4.2 and [67] (ZnO); band gaps: 2.17 eV[68] (Cu2O) and 3.44 eV(ZnO); dielectric constants:7.11(Cu2O) and 7.8(ZnO), electron effective mass:0.275mo(Cu2O) and hole effective mass in0.58 mo(Cu2O)-where mo is free electron mass.[68]
Transparent conducting metal oxides, being n-type were used extensively in the production of heterojunction cells using p-typeCu2O. Herion et al. (1980) have reported on a fairly detailed study of ZnO/Cu2Odevices. This was achieved due to the interest they had on metal oxides being generally stable compounds and the assumption that they are not likely to react withCu2O. The cell characteristics were clearly influenced by the copper rich region adjacent to Cu2O substrate. It was finally concluded that heterojunction is essentially Cu/Cu2O Schottky cell since Zn reduces Cu2O to Cu. Hence reaction occurs with this type of cells too.In all cases resistivity was found to be high and efficiency very low. However, the best solar cell, to date using TCO thin films is a multi-component oxides, ITO/ZnO/Cu2O solar cell. It was reported to have an efficiency of 2% (Mittiga et al., 2006). Copper (I) sulphide (Cu2S) is another promising material for heterojunction devices for the formation of n-Cu2O/ Cu2S heterojunction solar cell since Cu2S is a p-type semiconductor as grown. Works in the past were aimed at fabricating heterojunction devices. Fajinmi, (2000), reported on the deposition of Cu2S, while Varkey, (1990), reported on copper (I) sulphide/crystalline silicon (Cu2S/C -Si ) solar cells.
Homo-junction Solar Cells
In the past the low efficiency of Cu2O cells was attributed to the lack of n-type,since an approach to achieving n-type doping has not yet been fully developed. Without it, the early studies had to rely on Schottky junctions and p-n heterojunctions for photovoltaic devices, which do not provide high efficiency. However, Fernando et al, (2002), has reported the possibility of obtaining n-type photo-responses from clean copper plates, immersed in CuSO4 solution for a few days. Subsequently, the same researchersreported the n-type produced by heating copper sheets in CuSO4 solution.The long held consensus is that the best approach to improve cell efficiency in -based photovoltaic devices is to achieve both p- and n-type and thus p-n homo-junction of solar cells. This enables p-type and n-type Cu2O to be deposited electrochemically in sequence to form a p-n homo-junction of . However, the first homo-junction solar cell of using the electrochemical method was made by Kunhee and Meng (2009). The cell has only 0.1% efficiency due to high resistivity of the p- and n- layers (or substrate).
Cell characterization
Solar cell is a device that produces electricity from sunlight. Upon illumination, photons are absorbed by an active layer in a solar cell, and then electron-hole pairs are generated as excitons. The excitons need to diffuse to a donor–acceptor (DA) interface to dissociate into free charges. After that, electrons and holes need to transport to electrodes through their corresponding percolation pathway. During these processes, six main steps affect device performance:
Photon absorption (ηa),
Exciton generation (ηex),
Exciton diffusion (ηdiff),
Exciton dissociation (ηed),
Charge transport (ηtr),
Charge collection (ηcc),
These six processes determining a solar cell performance can be better understood in a connection with external quantum efficiency (EQE) of a device. is defined as a percentage of the number of charge carriers collected at the electrode under short-circuit condition to the number of photons incident on the device [68] EQE can be expressed as the product of the above steps.[69]EQE=ηa×ηex×ηdiff×ηed×ηtr×ηcc..(1)Solar cell efficiency can be calculated from its current density voltage (J – V) characteristic curves. From such curves, open-circuit voltage(Voc), short-circuit current density(Jsc) and fill factor (FF) can be obtained. Then energy conversion efficiency can be determined by:
Where is the incident light power densityEquivalent circuit of a solar cell is shown as Figure 6. A series resistance originates from contact and bulk semiconductor, and a shunt resistance comes from poor diode contact. The J-V characteristics can be described as
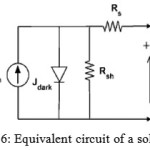 |
Figure 6: Equivalent circuit of a solar cell |
Equivalent circuit of a solar cell Where is Boltzmann’sconstant, is temperature, is elementary charge,is device area, is ideality factor of the diode, is reverse saturation current density, is photocurrent, Rs is series resistance and Rsh is shunt resistance.The J-V curves and photovoltaic parameters including and strongly depend on the n,, .In the mid-1970s, Cu2O gained renewed interest since it was considered as a low-cost material for solar cells. This renaissance took only about 10 years and the interest on Cu2O decreased. The developments made during that period are collected in the review of Rai from 1988[70]. It is worthwhile to have a look at his conclusions and suggestions for future work [70].
The poor performances were linked to a few but essential issues:(i) Control of the conductivity of the p-type layer by doping–an issue that has been solved by nitrogen doping. (ii) Schottky barrier solar cells have copper-rich or oxygen deficient surfaces that limit the performance and will always suffer from this problem (Schottky-type solar cells with Cu2Oare today no longer of relevance). (iii) Heterojunction solar cells should be investigated by which the chemical reactions at the interface between the two materials can be controlled and the reduction of Cu2O to Cu can be avoided. Indeed, in the last 8–10 years the investigations on cuprous oxide as a solar-cell material have put the focus on heterostructure systems and almost exclusively used ZnO as the n-type transparent window layer.Maximum values for the open-circuit voltage Voc are around0.87 V, short-circuit currents Isc around 10mA/cm2 and fill factors FF at.0.6The limitations in the performance were in part attributed to interface defects, crystal orientation, and grain sizes, series resistance due to the high resistivity of the absorber layer, and in recent reports to the minority-carrier transport length [71, 72]. It has been investigated that the open circuit voltage (Voc) and the short circuit current density (Ise) decrease with increase in temperature.(Voc) Drops because of increase reverse current saturation with temperature because minority carriers increase with increase in temperature.(Isc) Decrease because of increase the recombination of the charges.
Historical review on based Solar Cells
Even though Cu2Owas known since 1904, very little attention was paid to it. The few research works done on it were mainly concerning its photo-response, as reported [60]. By 1927 the transferring capacity of another metal-semiconductor junction solar cell, made of copper and the semiconductor copper oxide, had been demonstrated. Towards both the selenium cell and the copper oxide cell were employed in light sensitive devices, such as photometers for the use in photography. These early solar cells, however, still had energy-conversion efficiencies of less than 1%. As a result of pioneering work in 1975 at National Science foundation and at the Joint Centre for Graduate Studies (JCGS) on fabrication and characterization of Cu2O cells that Cu2O solar cells of 1 % efficiency were fabricated in1978. Further works on the cell at JCGS yielded an efficiency of 1.8% Experimental and theoretical studies conducted at JCGS group highlighted more on the potential viability of Cu2O for low cost photovoltaic (PV) power generation. Until recently, efforts in the past to fabricate efficient solar cells were confined to Schottky barrier solar cells. However, in recent times, single polycrystalline Cu2O used for solar cells are obtained via any of the methods presented earlier in this paper. The Schottky barrier solar cells and the heterojunctions have been extensively investigated. Also, in the past, workers have not succeeded in producing n-type and therefore a homo-junction cell structure could not be fabricated. Recently, Cu2O homojunction solar cells have been fabricated.
Performance of Cu₂O Solar Cells
The low efficiency was attributed to the naturally p-type conduction in Cu2O, formed a copper-rich region at the metallurgical interfacial region of Cu2O and other semiconductor or metal layer, which prevented a p-n junction in Cu2O, the basic device structure in most inorganic solar cells. In this case, a homo-junction of Cu2O is needed to achieve high efficiency Cu2O solar cell Both p-type and n-type Cu2O thin films prepared by electrodeposition have been reported by [73]they observed conversion efficiency of Cu2O cells has remained far below the theoretical value, regardless of the method of growth of Cu2O and the mode of fabrication of the cells. The best results obtained so far are in the range of 1-2% many reasons have been advanced for this low performance. Barrier height measurements in various Schottky barrier solar cells have shown that values are always in the range 0.7-0.9 eV regardless of the metal except for the case of gold and silver which form ohmic contacts with Cu2O.
This apparent plateau for the value of barrier heights is believed to be the principal cause of the low performance of the Cu2O Schottky barrier solar cells. Studies on Schottky barrier solar cell indicate that there always exist copper rich regions at the interface between metal and Cu2O regardless of the choice of the metal used. All Schottky type cells are therefore essentially Cu/Cu2O solar cells and hence the constancy of the value of barrier height and the low electrical power conversion efficiency. Papadimitriou et al. (1981)[74] have reported the results of their study on ZnO/Cu2O junction solar cells. The best values they obtained for open-circuit voltage (Voc) were of the order of 0.3 V. A copper rich region adjacent to the Cu2O substrate was found to be responsible for dictating the cell characteristics. For the case of Cdo/Cu2O heterojunction formed at room temperature, no copper metal was found at the interface.The Cdo/Cu2O cell showed a short circuit current of 2mAcm-2 and Voc of 0.4V. The method of electrodeposition is particularly attractive for its simplicity, low cost and possibility of making large area thin films [75]. But resistivity of electrodeposited Cu₂O films were reported to be quite high, of the order of 104-106 Ωcm(58, 76), and therefore,the photovoltaic properties of the solar cells made with these films were poor. Several other production techniques of Cu2O layer were later employed in order to improve on its resistivity.
The various methods include thermal oxidation (77-78), chemical vapor deposition, or radio frequency magnetron sputtering (79). However, little improvements were recorded as the efficiency was still very low, less that 2% Surface analyses combined with barrier height studies (59, 80) indicate that Cu2O Schottky barriers made with low work function metals are essentially Cu/Cu2O cells due to reduction of the Cu2O surface.The copper rich region essentially determines the barrier height.Auger depth profiles showed the occurrence of chemical reaction of thermodynamically more stable oxides at the interface and correspondingly to some reduction of the Cu₂O. Thallium is reported to be the only metal identified that would not reduce Cu2O(59). With 3.7eV as its work function and theoretical value of a dark current, Jo of the order of 10-8Acm10-2, a Tl/Cu2O Schottky barrier has a very large theoretical efficiency. Efficiency of 10% at air mass one (AM1) would be possible. However, deposition of Tl of reasonable sheet conductance is not possible on a substrate at room temperature.
It was reported that Tl of adequate sheet conductance was deposited on cool substrate. Studies on the Tl/Cu₂Obarrier represented only a slight improvement over the Cu/Cu₂O device. In fact studies on a number of metal/Cu₂O and metal/insulator/Cu₂O contacts (81) showed that the barrier heights did not depend on the work function of metal. Intensive work was done regarding deposition of Cu₂O and the dopant impurities (79, 82) but general observations show that the various dopants used to further lower the resistivity showed no significant improvement, with Cadmium as an exception. Nitrogen acts as a p-type dopant.
Other methods of improving on the resistivity of the layer were then employed. Annealing of the layer at moderate temperatures, as reported by [75, 83], showed some improvements. Another treatment followed; which is potassium cyanide (KCN) treatment as reported by[79]. The above treatments yielded little improvement since the efficiency, as at the time of this report, is still not more than 2%, for Schottky barrier Cu2O solar cells. The encouraging aspect of the above treatments is that, they revealed ways for further improvements. It was shown that the PV properties of Cu2O Schottky cells are significantly affected by the surface treatment and crystallinity of Cu2O [84]. In particular, the deposition method and conditions are important when depositing a thin film on Cu2O sheets.
Summary and outcome
In this report, we summarized the potential and possibilities of copper oxide semiconducting material, its band structure, method the production of Cuprous Oxide (Cu2O) and its applicability in solar cells.Some reasons based on the analysis reveals that (1) the high resistivity of starting material is responsible for the low value of electrical power conversion efficiency of Cu2O based solar cells. (2)Non–existence of a technique of doping Cu2O to get low resistivity n-type semiconductor before now so that conversion efficiencies greater 2% than for p-n homo-junction Cu2O solar cell could be fabricated. It is suggested that the work of Fernando et al, 2002 on production of n-type Cu2O, Long cheng and Meng, 2007 and Kunhee and Meng, 2009, on p-n Cu2O homojunction be pursued vigorously for the purpose of obtaining low resistivity Cu2O homojunction solar cells. (3) A copper rich or oxygen deficient surface which makes all Schottky barriers essentially a Cu/Cu2O structure. A suitable method to get rid of the cu-enriched region from the interface need to be explored.The copper oxide-based semiconductors that clearly fulfill the most important criteria of the future: availability, sustainability, non-toxicity (elements of hope), and ease of synthesis. It is therefore clear that a better understanding and remedy to the problems enumerated above demands more basic and applied investigation on Cu2O.
Acknowledgements
Special thanks to all members of Our Group for their useful discussion, cooperation, support and assistance in completing this Review article. The authors are grateful acknowledge to the University grant Commission (UGC) New Delhi, Government of India for support for this work.
Reference
- H. Y. Chen , J. Hou , S. Zhang , Y. Liang , G. Yang , Y. Yang , L. Yu , Y. Wu , G. Li , Nat. Photonics 2009, 3, 649
- B. O’Regan , M. Gratzel , Nature. 353, 737, (1991),
- C. Wadia , A. P. Alivisatos , D. M. Kammen , Environ. Sci. Technol. 43, 2072, (2009),
- T. G. Gutowski , M. S. Branham , J. B. Dahmus , A. J. Jones , A. Thiriez , Environ. Sci.Technol. 43, 1584, (2009).
- Y. Sheena Mary, C. Yohannan Panicker*,HemaTresa Varghese And Somi Sebastian., Oriental Journal Of Chemistry Vol. 26(3), 901-910 (2010)
- K. Anuar , Z. Zainal , M. Z. Hussein , N. Saravanan , I. Haslina , Sol. Energy Mater. Sol. Cells , 73,351. (2002).
- L. Wang , K. Han , M. Tao , J. Electrochem. Soc. 154, D91 , (2007).
- D.K. Zhang, Y.C. Liu, Y.L. Liu, H. Yang, Physica B 351 , 178 (2004).
- A.E. Rakhshani, Solid State Electron. 29 , 7 (1986).
- H. Raebiger, S. Lany, A. Zunger, Phys. Rev. B 76 , 045209 (2007).
- PeiweiLv, WeifengZheng, Limei Lin, FuchuanPeng, Zhigao Huang, Fachun Lai, Physica B 406 , 1253–1257 (2011).
- G.P. Pollack, & D. Trivich, Journal of Applied Physics,.46:1, 163–172 (1975).
- P. E. de Jongh , D. Vanmaekelbergh , J. Kelly , Chem. Mater. , 11, 3512 , (1999).
- K. Mizuno , M. Izaki , K. Murase , T. Shinagawa , M. Chigane , M. Inaba , A. Tasaka , Y. Awakura , J. Electrochem. Soc. , 152, C179 , (2005).
- C. Noguet, M. Tapiero, C. Schwab, J.P. Zielinger, D. Trivich, R.J. Komp, E.Y. Wang, and K.,Wang, 1 st European community Photovoltaic conference proc. P. 1170, (1977).
- B. K. Meyer,et.al., Phys. Status Solidi B249, No. 8, 1487–1509 (2012)
- Hideki Ushio, Shunichi Matsuno, Tsuyoshi Hamada., Theory of Copper Oxide Superconductors. Springer London, 214, 2005
- Cuprite —Mindat directory (2010).
- A. F. Wells, Structural Inorganic Chemistry, 3rd edn (Clarendon Press, Oxford, 1984)
- J. P. Dahl and A. C. Switendick, J. phy. Chem. Solids, 27,931-942,(1966)
- R. J. Elliot, J. phys. Rev.,124, 340-345, (1961)
- E. F. Gross, IlNuovo Cimento3, 672 (1956).
- E. F. Gross and F. I. Kreihgol’d, JETP Lett.7, 218 (1968).
- E. F. Gross and F. I. Kreihgol’d, JETP Lett.10, 139 (1969).
- J. B. Grun, M. Sieskind, and S. Nikitine, J. Phys. Chem. Solids19, 189 (1961).
- R. J. Elliott, Phys. Rev. 124, 340 (1961).
- R. J. Elliott, Phys. Rev. 108, 1384 (1957).
- J. P. Dahl, J. Phys. Colloq. 28, C3 (1967).
- J. P. Dahl and A. C. Switendick, J. Phys. Chem. Solids27, 931 (1966).
- A. Daunois, J. L. Deiss, and B. Meyer, J. Phys. 27, 142 (1966).
- M. Balkanski, Y. Petroff, and D. Trivich, Solid State Commun.5, 85 (1967).
- V. T. Agekyan, Phys. Status Solidi A43, 11 (1977).
- R. A. Forman, W. S. Brower, Jr., and H. S. Parker, Phys. Lett. A36, 395 (1971).
- D. Frohlich, R. Kenklies, C. Uihlein, and C. Schwab, Phys. Rev. Lett.43, 1260 (1979)
- W. H. Brattain, Rev. Mod. Phys. 23, 203 (1951).
- C. Carel, M. Mouallem-Bahout, and J. Gaude ´, Solid State Ion.117, 47 (1999)
- W.M. Sear, E.J. Fortin, Solar Energy materials, 10: 93 – 103 (1984)
- D. Wu, Q. Zhang, and M. Tao, Phys. Rev. B 73, 235206(2006).
- H. Raebiger, S. Lany, and A. Zunger, Phys. Rev. B 76,045209 (2007).
- A. F. Wright and J. S. Nelson, J. Appl. Phys. 92, 5849(2002).
- M. Nolan and S. D. Elliott, Phys. Chem. Chem. Phys. 8,5350 (2006).
- D. O. Scanlon and G. W. Watson, J. Mater. Chem.21, 3655(2011).
- M. A. Meki-Jeskari, Model. Simul.Mater.Sci. Eng.14, 207(2006).
- D. O. Scanlon, B. J. Morgan, and G. W. Watson, Phys. Rev.Lett.103, 096405 (2009).
- Y. S. Lee, M. T. Winkler, S. Cheng Siah, R. Brandt, and T. Buonassisi, Appl. Phys. Lett.98, 192115 (2011).
- H. L. MicKinzie and M. O’Keeffe, Phys. Lett. 24A, 137 (1967).
- G. P. Pollack and D. Trivich, J. Appl. Phys.46, 163 (1975).
- M. Zouaghi, M. Tapiero, J. P. Zielinger, and R. Burgraf, Solid State Commun.8, 1823 (1970).
- H. Shimada and T. Masumi, J. Phys. Soc. Jpn. 58, 1717 (1989).
- J. Bloem, A. J. Van der Houven van Oordt, and F. A. Kroger,Physica22, 1254 (1956).
- MehranRiazianAnd Ali Ramzannezhad., Oriental Journal Of Chemistry Vol. 28, No. (1):Pg. 73-82 (2012),
- C. Duvvury, D. J. Kenway, and F. L. Weichman, J. Lumin.10, 415 (1975).
- H. Solache-Carranco, G. Juarez-Dıaz, A. Esparza-Garcıa, M. Briseno-Garcıa, M. Galvan-Arellano, J. Martınez-Juarez, G. Romero-Paredes, and R. Pen a-Sierra, J. Lumin.129, 1483 (2009).
- T. Ito and T. Masumi, J. Phys. Soc. Jpn.66, 2185 (1997)
- Musa, A.O., Ph.D Thesis, University of Ilorin, Nigeria. 108-136.,(1995):
- Trivich, D., Wang, E.Y. Komp, R.J. Kakar, A.S.,13th IEEE Photovoltaic Specialist Conf. Proc. 174., (1978)
- N.A. Economou, R.S. Toth, R.J. Komp and D. Trivich., 14th IEEE Photovoltaic Spec. Conf. Proc. New York: 1180-1185., (1982).
- L.C. Olsen, F.W. Addis and R.C. Bohara., 14th IEEE photovoltaic Specialist Conf. Proc., IEEE, New York, , p. 462. (1980)
- L.C .Olsen, F.W. Addis, and W. Miller., J. Sol. Cells,7: 247 – 279. (1982).
- EsmaeilBiazar*, BehnamBaghermanesh And K. SaeedHeidari., Oriental Journal Of Chemistry Vol. 27, No. (3), 953-958 (2011),
- D.P.Singh, J. Singh, P. R. Mishra, R. S. Tiwari, O. N. Srivastava., J. Bulletin of Materials Science, 31:3, pp. 319–325, (2008).
- V.F. Drobny, and D.L. Pulfrey., J.Thin solid films,61: 89-98., (1979)
- H. Kobayashi, T. Nakamura, & N. Takahash., J. Materials Chemistry and Physics, 106:2-3, pp. 292-295, (2007).
- M. Izaki, T. Shinagawa, K. Mizuno, Y. Ida, M. Inaba, and A. Tasaka., J.Phys.D:Appl.Phys.40 3326-3329. (2007).
- S. S. Jeong, A. Mittiga, E. Salza, A. Masci, S. Passerini, Electrochim. Acta 53, 2226(2008).
- H. Kobayashi, H. Mori, T. Ishida, Y. Nakato, J. Appl. Phys., 77, 1301 (1995).
- V.A. Modhavadiya., Oriental Journal Of Chemistry Volume No. 28 Issue No.: 2 Page No. 921-925 June-Dec (2012).
- Mohammad Ramzan Parra, PadminiPandey, Neha Singh, Hafsa Siddiqui and Fozia Z. Haque, journal of material sciences, Volume No.9 Issue No.: 1, June (2012).
- AlirezaJafari, MasoodGhane*, MehrdadSarabi And FarhodSiyavoshifar4., Oriental Journal Of Chemistry Vol. 27, No. (3), 811-822 (2011)
- B. P. Rai, Solar Cells 25, 265 (1988).
- T. Minami, Y. Nishi, T. Miyata, and J.-I. Nomoto, Appl. Phys. Express 4, 062301 (2011).
- Y. Liu, H. K. Turley, J. R. Tumbleston, E. T. Samulski, and R. Lopez, Appl. Phys. Lett. 98, 162105 (2011).
- B.P. Rai, J.Solar Cells, Pages 265–272 (1988)
- L. Papadimitriou, N.A. Economou, and D. Trivich., J. Solar cells, 3: 73 – 80. (1981).
- R.P. Wijesundara, M. Hidaka, K. Koga, and W. Siripala., J.Thin solid films500: 241 – 246., (2006)
- R.P. Wijesundara, L.D.R.D. Perera, K.D. Jayasuriya, W. Siripala., K.T.L. De Silva, A.P. Samantilleke I.M. Dharmadasa., J. Solar energy materials and solar cells, 61: 277-286., (2000).
- KumariSapna1 ,Navin Kumar Sharma2 And Seema Kohli1., oriental journal of chemistry volume no. 28 issue no.: 2 page no. 969-974 june-dec (2012).
- W.M. Sear, and E.J. Fortin., J. Solar Energy materials,10: 93 – 103, (1984).
- A. Mittiga, A. Salza, E. Sarto, F. Tucci, M. and Vasanthi, R., J. Applied physics letters, 88: 163 502-1 – 163502-2, (2006).
- K.Akimoto, S. Ishizuka, M.Yanagita, Y.Nawa, K.G. Paul, T. Sakurai., J. Solar energy, 80: 715 – 722., (2006).
- MehranRiazian*, NaserMontazeri and EsmaeilBiazar., Oriental Journal Of Chemistry Vol. 27, No. (3) 903-910 (2011)
- A. O. Musa, T. Akomolafe, and Carter, M. J., Solar energy materials and solar cells, Pergamon, 51: 3-4. (1998)
- H. Tanaka, T. Shimakawa, Miyata, T. Sato, H. Minami, T., J. Thin solid films, 469-470: 80 -85.(2004).
- C.A.N. Fernando, P.H.C. de Silver, S.K. Wethasinha, I.M. Dharmadasa, T. Delsol, & M. C. Simmonds, J. Renewable energy, 26: 521-529.,(2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.











