The One-Pot Three Components of Iodone, Schiff Base and Epoxide for Synthesis of Iodohydrins
Abolghasem Shameli1*, Abdol Hamid Raeisi2, Bahram Pourhasan3*, Hossein Naeimi4* and Mohammad Mehdi Ghanbari5
1Department of Chemistry, Islamic Azad University Omidiyeh branch, Omidiyeh, (Iran). 2Department of Chemistry, Technical and Vocational University Amozeshkade Pesaran Gorgan, Gorgan, (Iran). 3Department of Chemistry, Islamic Azad University Dezful branch, Dezful, (Iran). 4Department of Chemistry, Kashan University, Kashan, (Iran). 5Department of Chemistry, Islamic Azad University Sarvestan branch, Sarvestan, (Iran).
In study we report synthesis Iodohydrines in absence of the complexes Schiff-base Salen Cu, Ni and Co. The experimental results showed both that the catalysts had higher catalytic activity and better epoxide selectivity than the homogeneous catalyst.
KEYWORDS:Schiff base; Iodohydrin; Ligand; Iodine; Homogeneous
Download this article as:| Copy the following to cite this article: Shameli A, Raeisi A. H, Pourhasan B, Naeimi H, Ghanbari M. M. The One-Pot Three Components of Iodone, Schiff Base and Epoxide for Synthesis of Iodohydrins. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Shameli A, Raeisi A. H, Pourhasan B, Naeimi H, Ghanbari M. M. The One-Pot Three Components of Iodone, Schiff Base and Epoxide for Synthesis of Iodohydrins. Available from: http://www.orientjchem.org/?p=23387 |
Introduction
With the increasing application of rare earth metals in a variety of fields, rare earth ions continually intrude into general environment and further into the bodies of plants, animals and human beings. It is therefore of significance to investigate the physiological action and long-term effect of rare earth ion on biological bodies. Various studies have shown that Schiff bases derived from Salicylaldehyde and its derivatives have considerable biological importance partly because such ligands have many donor atoms (N, 0) and are analogous to biological environment to some extent. They have been widely used in the fields of biology, pharmacology, catalysis, organic synthesis, chemical, analysis, and so on.1-4much attentions have been paid to these Schiff bases because of the stability of the ligands and various properties of their metal complexes.5-10
As an important strategy for the formation of 1,2-bifunctionalized chiral building blocks, the enantioselective ringopening of epoxides with different nucleophiles has attracted much attention from the organic chemists. A wide variety of nucleophiles, such as alcohols, phenols, carboxylic acids, amines, azide ions, thiols, cyanide ions and halide ions are utilized in the aforementioned reaction.11
In continuation of our work on enantioselective epoxidation12-17 of non-functionalized olefins by chiral Co(II), Ni(II) and Cu(II) Schiff base complexes and in pursuit of better selectivity through electronic tuning in the catalyst, we are reporting here the applying catalyst in ring opening opexides.
Experimental:
All of the reagents were supplied by Merck and Fluka, and were employed without further purification. IR spectra were recorded on a Unicam Matson 1000 FT-IR paragon 1000 spectrophotometer. 1H NMR spectra of the ligand and the complex were recorded on a Bruker FT-NMR 500 MHz spectrometer using CDCl3 and (CD3)2SO as solvents. The electronic spectra were recorded on a CARY 100 Bio UV–Vis spectrophotometer.
General Procedure for Synthesis of 2,2′-[1,2-Ethandiyl bis(nitrilopropelidene))]bis(1-naphthol): To the stirred solution of 1-(2-hydroxynaphthalen-3-yl)propan-1-one (4 mmol) in 5ml MeOH Ethylendiamine (2 mmol) was added at room temperature. The reaction was continued for 3.4 h. The progress of the reaction was monitored by TLC. After the reaction was completed, the brown oil was collected and dissolved in hot petroleum ether. After cooling pail yellow solid product was obtained. The precipitate was filtered off and washed with cold MeOH. The crude product was purified by recrystallization in ethanol and the pure Schiff base, 2,2′-[1,2-Ethandiyl bis(nitrilopropelidene))]bis(1-naphthol) was obtained in 92% yield, m.p=196-198oC. The structure of the Schiff base was confirmed by physical and spectroscopic methods
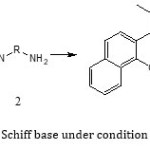 |
Scheme1: synthesis Schiff base under condition room temperature |
2,2′-[1,2-Ethandiyl bis(nitrilopropelidene)]bis(1-naphthol) (3a): Pail yellow, M.P=196-198 0C , IR(KBr)/ ν (cm-1): 3550-3300 )OH), 1600 )C=N), 1540 (C=C, Ar), 1H NMR/CDCl3 δ p.p.m; 1.2-1.6(6H, t, J=7.2), 3(4H, dd, J=7.2) ,4.2 (4H, s), 7-8.4 (10H ,m), 8.6 (2H, d), 16.8( 2H, s), λmax: 322 nm.
2,2′-[1,3-propandiyl bis(nitrilopropelidene)]bis(1-naphthol) (3b): Pail yellow, M.P=196-198 0C , IR(KBr)/ ν (cm-1): 3250-3550)OH), 1600 ) C=N), 1540 (C=C, Ar), 1H NMR/CDCl3 δ p.p.m; 1.2-1.6(10H, m), 3 (4H, m) ,4.2 (4H, s), 7-8.4 (10H ,m), 8.6 (2H, m), 16.8( 2H, s), λmax: 330 nm.
Preparation of the Complexes:
Ethanolic solutions of metal acetate (0.025mol) and schiff base (0.05mol) were mixed and the resulting mixture under conditions N2, until the metal Salen separated, which were then suction filtered, washed with ethanol and ether before dried in vacuum dessicator. The crystals were recrystallized from rectified sprit and dried (Mahaptra et al., 1977) (scheme1).
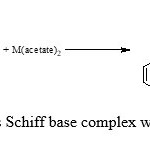 |
Scheme2: synthesis Schiff base complex with Co, Cu and Ni |
2,2′-[1,2-Ethandiyl bis(nitrilopropelidene)]bis(1-naphtholate) Copper(II) (4a): solid brown, M.P=335-3370C , IR (KBr)/ ν (cm-1): 1630 ) C=N), 1550 (C=C, Ar), λmax: 318 nm
2,2′-[1,2-Ethandiyl bis(nitrilopropelidene)]bis(1-naphtholate) Cobalt(II) (4b): solid brown, M.P=337-33390C , IR (KBr)/ ν (cm-1): 1635 ) C=N), 1560 (C=C, Ar), λmax: 320 nm
Synthesis bromohydrin by catalyst 2,2′-[1,2-Ethandiylbis (nitrilopropelidene)]bis(1-naphth-olate) Cobalt(II)
Epoxide (1 mmol) in CH2Cl2 (5 mL) was added to a stirred 2,2′-[1,2-Ethandiyl bis(nitrilo propelidene)]bis(1-naphtholate) Cobalt(II)catalyst (0.05 mmol) in at room temperature. Next, a solution of elemental iodine (1 mmol) in CH2Cl2 (5 mL) was added portion-wise (15 min) to the above mixture. The progress of the reaction was monitored by TLC. After complete disappearance of the starting material, the reaction mixture was washed with 10% aqueous Na2S2O3 (2×10 mL) and water (2×10 mL). The aqueous layer was extracted with CH2Cl2 (2×10 mL).The combined organic layer was dried over anhydrous MgSO4 and evaporated to give crude alcohol–catalyst (scheme3).
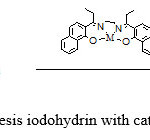 |
Scheme 3: synthesis iodohydrin with catalyst Schiff base |
1-Iodo-2-boutanol (99%): 1HNMR (CDCl3, 300MHz) δ 0.95 (t, 3H, J=7.2Hz), 1.55-1.7 (m, 2H), 3.05-3.25(m, 1H), 3.3-3.5 (m, 1H), 3.75-3.8(m, 1H).; MS(EI) M/Z 200( M+)
2-Iodo Cyclohexanol (97%): 1HNMR (CDCl3, 300MHz) δ 1.2-1.6(m, 4H), 1.8-1.9 (m, 1H), 2.0-2.2(m, 2H), 2.4-2.5(m, 1H), 3.5-3.65(m, 1H), 3.95-4.05(m,1H);MS(EI) M/Z 226( M+); IR(KBr) 3425, 2960 cm-1
2-Iodo-1-(4-Cholrophenyl)ethanol (96%): 1HNMR (CDCl3, 300MHz) δ 2.45(br, 1H), 3.45-3.5 (m, 2H), 4.80-4.88(m, 1H), 7.2-7.4(m, 4H);MS(EI) M/Z 282( M+); IR(KBr) 3460, 2960 cm-1
2-Iodo-1-phenyl ethanol (100%): 1HNMR (CDCl3, 300MHz) δ 2.50(br, 1H), 3.39-3.5 (m, 2H), 4.75(m, 1H), 7.25-7.4(m, 5H);MS(EI) M/Z 248( M+); IR(KBr) 3398 , 2960 cm-1
1-Iodo-3-(4-methoxyphenyl)2-propanol (99%): pale yellow liquid, 1HNMR (CDCl3, 300MHz) δ 2.05(br, 1H), 2.85(d, 2H, J=6.2, 9.2Hz), 3.25 (dd, 1H, J=4.8,9.2 Hz), 3.35 (dd, 1H, J=3.8,9.2Hz), 3.6-3.75(m, 1H), 3.80(S, 3H),6.85(d, 2H,J=8.2Hz), 7.15(d, 2H, J=8.2Hz); 13CNMR (CDCl3,50Hz) δ 14.67, 41.66, 55.10, 71.62, 113.92, 128.98, 130.12, 158.25;MS (EI) M/Z 292 (M+); IR(KBr) 3560, 3050, 2960 cm-1
1-Iodo-3-(4-acetylphenoxy)2-propanol (99%): yellow Solid, mp 68-700 C, 1HNMR (CDCl3, 300MHz) δ 2.55(S, 3H), 3.30-350 (m,2H), 3.90-4.05 (m, 3H), 6.90(d, 2H,J=7.8Hz), 7.9(d, 2H, J=7.8Hz);13CNMR (CDCl3,50Hz) δ 8.84, 26.21, 69.12, 70.15, 114.66, 130.64, 162.75, 196.96; MS (EI) M/Z 320 (M+); IR(KBr) 3460, 3020, 2970, 1710 cm-1
1-Iodo-3-(4-Cholorophenoxy)2-propanol (98%):1HNMR (CDCl3, 300MHz) δ 2.40 (br, 1H), 3.35-3.40 (m,2H), 3.45-3.50 (m, 1H), 3.95-4.0 (m, 1H), 4.05-4.10(m, 2H), 6.85(d, 2H, J=8.2 Hz), 7.15(d, 2H, J=8.2 Hz); MS (EI) M/Z 312 (M+); IR(KBr) 3515 cm-1
1-Iodo-3-phenoxy-2-propanol(97%): 1HNMR (CDCl3, 300MHz) δ 2.40(br, 1H), 3.30-3.55 (m, 2H), 3.8-4.1(m, 3H), 6.75-7.0(m, 3H) 7.15-7.35(m,2H);MS(EI) M/Z 278(M+); IR (KBr) 3500 , 2985 cm-1 .
Result and Discussion:
As shown in Scheme 1, when 2 mols heterocyclic ketone were treated with 1 mol diamine at room temperature, a pail yellow substance obtained with high yield. In this reaction, heterocyclic ketones have been applied and corresponding products were obtained. The results and conditions of the reactions are presented in Table 1.
As can be seen in our previously reported works on synthesis of Schiff bases from ortho-hydroxy aldehyde [14-16] and ortho-hydroxyl ketene, [16-17] the presence of hydroxyl group in ortho situation is accelerated condensation reaction. While, in used heterocyclic aldehydes, with respect to absence of o-hydroxy group, the corresponding Schiff bases were obtained in high yields and appropriate reaction times (Table 1, Entries 1, 2).
 |
Table 1: Preparation of Schiff base containing heterocyclic rings through two component reaction |
All the new, potentially hexadentate Schiff base ligands were cleanly synthesized in 1-1.5 minutes and >80% yield according to elemental analyses and 1H and 13C NMR analyses of the bulk products after recrystallization from ethanol (Table 1). Their structures are supported by the absence from their IR spectra of the carbonyl and primary amine bands of the reagents, and the presence of a Schiff base ν(C=N) band in the 1631-1652 cm-1 region; the alkyl C–H stretching vibrations appear in the 2800–2900 cm−1 region. In the 1H NMR spectra, the azomethine protons appear at δ= 8.22–8.73 ppm and the aromatic ring protons at δ = 6.5-8.4 ppm.
In conjunction with ongoing work in our laboratory on the synthesis and formation of complex heterocyclic compounds containing donor nitrogen atoms, with neutral molecules such as bromine, we found out that 2,2′-[1,2-Ethandiylbis(nitrilopropelidene)]bis(1-naphth-olate) Cobalt(II)with frame nano efficiently catalyzed the addition of elemental bromine to epoxides under mild reaction conditions with high regioselectivity (Scheme 3).
In this study, we wish to report the results of the reactions of some styrene oxide with elemental bromine in the presence of a sub-stoichiometric 0.01 mmol amount of catalyst Schiff base (Scheme 3, Table 2).
Table 2: Amount of Catalyst in Ring Opening Epoxide
|
Entry |
Complex Schiff base |
Yielda (%) |
Entry |
Complex Schiff base |
Yielda (%) |
Entry |
Complex Schiff base |
Yielda (%) |
|
1 |
4a |
40 |
8 |
4h |
15 |
15 4q 10 |
||
|
2 |
4b |
70 |
9 |
4k |
10 |
16 4r 5 |
||
|
3 |
4c |
25 |
10 |
4l |
10 |
17 4s 5 |
||
|
4 |
4d |
30 |
11 |
4m |
20 |
18 4t 10 |
||
|
5 |
4e |
15 |
12 |
4n |
10 |
19 4w 5 |
||
|
6 |
4f |
20 |
13 |
4o |
10 |
20 4x 5 |
||
|
7 |
4g |
10 |
14 |
4p |
10 |
21 4y 5 |
||
aIsolated product yields
Then in this article, we report ring opening styrene oxide with amount deferent of catalyst 4b, that the results of reaction showing in table 3.
Table 3: cleavage styrene oxide with amount deferent of catalyst 4b
|
Entry |
Complex 4b (% mol) |
Yielda (%) |
Entry |
Complex 4b (% mol) |
Yielda (%) |
Entry |
Complex 4b (mol) |
Yielda (%) |
|
1 |
1 |
70 |
4 |
4 |
90 |
7 7 100 | ||
|
2 |
2 |
75 |
5 |
5 |
98 |
8 10 100 |
||
|
3 |
3 |
80 |
6 |
6 |
98 |
9 15 100 | ||
aIsolated product yields
The crude products were purified on a column of silica gel. The solvent was evaporated and pure iodohydrin was obtained. The iodohydrins obtained throughout this procedure were identified by comparison, where possible, with authentic samples prepared in accordance with literature procedures.
Conclusion
In conclusion, this new method appears to be highly competitive with the other methods reported in the literature. The reaction occurs in neutral and mild conditions on the acid-sensitive substrates and vicinal iodohydrins were obtained in high yields and regioselectivity. In addition, in comparison with our previous methods, 2,2′-[1,2-Ethandiylbis(nitrilopropelidene)] bis(1-naphtholate) Cobalt(II) is cheaper, less step need for preparation, and overall yield is higher
Acknowledgment
This study financially supported by Islamic Azad University Dezful branch.
Reference
- Dey K. Schiff bases and their uses [ J ] . J . Scient . Ind. Res., 1974, 33: 76.
- Dhar D N , Taploo C L. Schiff bases and their applications [J]. J. Scient. Ind. Res., 1982, 41: 501.
- Liu, X. L.; Liu, Y. H.; Shi, Y. C.; et al. Application of Schiff’s bases in organic synthesis [J] . Chinese Journal of Organic Chemistry (in Chin.), 2002, 22(7): 482.
- Hodnett , E .M.;Dunn, W. J. Structure-antitumor activity correlation of some Schiff bases [J]. J. Med. Chem., 1970, 13(4): 768.
- You, X .Z.; Meng, Q. J.; Han, W. S. Progress in Coordination Chemistry [ M ] . Beijing : Higher Education Press, China,. 2000, 24.
- Costes, J.P.; Dahan, F.; Nicodene , F. Structure-based description of a step-by-step synthesis of homo- and heterodinuclear (4f, 4f’) lanthanide complexes [J]. Inorg Chem., 2003, 42: 6556.
- Golcu A, Turner M, Demireli H, et al. Cd (II) and Cu (II) complexes of polydentate Schiff base ligands synthesis, characterization, properties and biological activity [J]. Inorg. Chim. Acta., 2005, 358: 1785.
- Vigato P A, Fenton D E . Schiff base complexes of lanthanides and actinides [J] . Inorg. Chim . Acta, 1987, 139: 39.
- Wu Z S. A Survey of the study of Schiff base metal complexes as anti-cancer agents [ J ]. Journal of Central China Normal University ( Nat. Sci. Ed. ) , 1983, 17 ( 1 ) : 61.
- Zhang X Y, Zhang Y J, Yang L. Progress in studies on rare earth Schiff base complexes in our country [J], Chemical Researh and Application, 2002, 14 (1): 9.
- Pastor, I.M. us, M. Y. Asymmetric Ring Opening of Epoxides. Curr. Org. Chem. 2005, 9, 1-29
- Hodgson, D.M. Gibbs, A.R. Lee, G.D. Enantioselective desymmetrisation of achiral epoxides. Tetrahedron, 1996, 52 14361
- Paterson, I. Berrisford, D.J. (Organoarsonato)polyoxovanadium Clusters: Properties and Structures of the VV Cluster [V10O24(O3AsC6H4-4-NH2)3]4− and the VIV/VVCluster [H2{V6O10(O3AsC6H5)6}]2−, Angew. Chem., Int. Ed. Engl,. 1992, 31 1197.
- Sharghi, H.; Naeimi, H. Schiff-Base Complexes of Metal(II) as New Catalysts in the High-Regioselective Conversion of Epoxides to Halo Alcohols by Means of Elemental Halogen,Bull. Chem. Soc. Jpn. 1999, 72,1525.
- Naeimi, H.; Moradian M. Metal(II) Schiff base complexes as catalysts for the high-regioselective conversion of epoxides to β-hydroxy nitriles in glycol solvents, Can. J. Chem. 2006, 84, 1575.
- Naeimi, H.; Salimi F.; Rabiei, Kh. Mild and convenient one pot synthesis of Schiff bases in the presence of P2O5/Al2O3 as new catalyst under solvent-free conditions. J. Mol. Catal. A, Chem. 2006, 260, 100.
- a) Naeimi, H.; Sharghi, H.; Salimi F.; Rabiei, Kh. Facile and efficient method for preparation of schiff bases catalyzed by P2O5/SiO2 under free solvent conditions. Heteroatom Chem. 2008, 19, 43.b) Shameli, A.; Ghanbari, M. M. Ring Opening Epoxides into Iodohydrins with Elemental Iodine in The Presence of Catalyst Nano Powder ZrO2. Trends in Modern Chemistry, 2012, 3(1), 14-17.

This work is licensed under a Creative Commons Attribution 4.0 International License.









