Synthetic Approaches and Electronic Spectra of Some New Butamethine Asycyanine Colorants
Bhupendra Narayan1, Mobashshir Hasan Khan, Imtiaz Ali, M. Wayzul Haque and A.S. Ansari2
1G.D. College Begusarai- 851 101 (India). 2University Department of Chemistry, L.N.M. U. Darbhanga - 846 004, (India).
Fifteen new chromophoric chain ?-substituted butamethine asycyanine (CCBSBA) colorants have been synthesized by catalytic condensation of (i) 4- Dimethylaminostyrylphenyl ketone ; (ii) 4- Dimethylaminostyryl-3’- nitrophenyl ketone and (iii) 4-Dimetjylaminostyryl-3’-methylphenyl ketone with 2-methyl-3-(1-methylethyl)benzothiazolium iodide and 2-methyl-6-substituted-3-(1-methylethyl)benzothiazolium iodides using piperidine as basic catalyst and ethanolic DMF as solvent. These colorants were synthesized with the object to study the impact of various functional groups and chain enhancement and shortage of prime chain on visible electronic spectra. All the colorants showed increase in electronic spectra whether electron withdrawing or donating. These colorants led to red shifts of the electronic spectra w.r.t. the previously reported styryl chain asycyanino-colorants, and to blue shifts of electronic spectra w.r.t. previously reported asycyanine colorants having quinoline moiety.
KEYWORDS:Benzothiazole; Asycyanine Colorants; Dimethylaminostyrylphenyl ketone; Benzothiazolium iodides; Electronic spectra; Red shift; Blue Shift
Download this article as:| Copy the following to cite this article: Narayan B, Khan M. H, Ali I, Haque M. W, Ansari A. S. Synthetic Approaches and Electronic Spectra of Some New Butamethine Asycyanine Colorants. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Narayan B, Khan M. H, Ali I, Haque M. W, Ansari A. S. Synthetic Approaches and Electronic Spectra of Some New Butamethine Asycyanine Colorants. Available from: http://www.orientjchem.org/?p=23468 |
Introduction
Due to multipurpose applications of cyanine, polymethinecyanine and asycyanine colorants they are being used in cosmoelectronic photography , laser technology, solar energy conversion systems1-3, absorptivity and photo sensitivity4-17. Hence they became more vital in research field. For this purpose fifteen chromophoric chain β -aryl substituted butamethine benzothiazole asycyanine colorants were synthesized by catalytic condensation of 2-methyl-3-(1-methylethyl)benzothiazolium iodide and 2-methyl-6-substitued-3-(1-methylethyl)benzothiazolium iodides with 4-Dimethylaminostyrylphenyl ketone, 4-Dimethylaminostyryl-3’-nitrophenyl ketone and 4-Dimethylaminostyryl-3’-methylphenyl ketone using piperidine as basic catalyst and ethanolic DMF as solvent (scheme-1).
These colorants were synthesized with the aim:
(i)To study the effect of electron donor and electron acceptor additives at the 3- position in the chain β-phenyl nucleus & at the 6-position of heterocyclic system.
(ii)To study the effect of electron donor and electron acceptor additives present at the 3-position w.r.t. electron donor and electron acceptor additives present at the 4-position in the chain β-phenyl nucleus and at the 6-position of the heterocyclic system.
(iii)To study the electronic spectra of synthesized colorants with reported asycyanine colorants whose length are shorter i.e. w.r.t. styryl colorants with reported asycyanine colorants whose length are longer i.e. w.r.t. hexatrienyl quinaldine system.
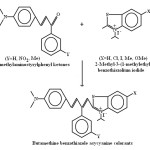 |
Scheme 1 Click here to View scheme |
EXPERIMENTAL
Synthesis of 2-Methyl-3-(1-methylethyl)benzothiazolium iodide and four 2-methyl-3-(1-methylethyl)-6-substituted(chloro, iodo, methyl, methoxy)benzothiazolium iodide were synthesized by earlier methods3 with some procedural alteration.
Synthesis of complex auxochromic ketones:
4-Dimethylaminostyrylphenyl ketone (reported) and 4-Dimethylaminostyryl-3-substituted (nitro, methyl,) phenyl ketones were synthesized by usual process4-6using N,N-Dimethylaminobenzaldehyde,acetophenone, and 3-substituted (nitro,methyl)acetophenone.
4-Dimethylaminostyrylphenyl ketone:
The crude product was recrystallised from ethanol as bright yellow leaf.
Yield 69% m.p. 1100C
(Lit. yield 70%, m.p. 1100c)8
4-Dimethylaminostyryl-3-nitrophenyl ketone:
The crude product was recrystallised from ethanol as deep red crystals.
Yield-80%m.p. 152-1540C
Found : C- 68.88%; H-5.42%; N-9.44%
IR Spectra: KBr (Cm-1)1610 (CH=CH)
1695 (C=O), 1615 (C=N),
1590 (NO2)
4-Dimethylaminostyryl -3-nitrophenyl ketone.
The crude product was recrystallised from ethanol as turmeric yellow sandy crystals.
Yield: 60% m.p. 710C
Found : C- 81.45%; H- 7.20%; N- 5.25%
IR Spectra: KBr (Cm-1) 1610 (CH=CH)
1680 (C=O) 1617 (C=N)
3010 (CH3)
Synthesis of CCBSBA Colorants:
The condensation to obtain the colorants were carried out by earlier methods with some modification 7.8.
A solution containing the quaternised salt and complex auxochromic ketone in milli molar ratio in ethanolic DMF (25ml) in the presence of basic catalyst piperidine (2-3 drops) was refluxed for 6-8 hrs under anhydrous conditions using CaCl2 guard tube. The resulting mixture was concentrated, cooled and left overnight at room temperature. The afforded colorant was recrystallised from methanol. The analytical and UV spectral data of the colorants are given in table 1.
Table 1: Analytical & Spectral data of the Colorants
| Colorants | Yield% | m.p.(oC) |
% Found (calcd) |
Crystal, Shape & Color | λmax(nm)(in abs. EtOH) | PMR,p.p.m.Δ (Ar-H) | Vmax(cm-1) | |
|
N |
X |
|||||||
| Series I (X=H, Cl, I, Me, OMe; Y= H) | ||||||||
| C1 | 19 | 205 | 5.00(5.07) | 22.92(23) | y’bc | 410 | 7.01-7.02 | 2930-3020 C-H (Str.) (Aromatic)2410-2450 C=N(str.)(Quaternary N)
1390-1630 C=C(Str.) (Aromatic & conjugation with C=N,plane vibration) 730-880 C-H (Def) (Aromatic nucleus) 500-770 C-X (Str.),-Cl,- I
3080-3110 C-H (Str.) (Aromatic) 2420-2460 C=N (Str.) (Quaternary N) 1420-1660 C=C (Str.) (Aromatic & conjugation with C=N plane vibration) 1300-1370 N=O(Str.) 745-875 C-H (Def) (Aromatic nucleus) 500-770 C-X(Str.), -Cl, -I
2990-3030 C-H (Str.) (Aromatic) 2410-2460 C=N (Str.) (Quaternary N) 1410-1660 C=C (Str.) (Aromatic & conjugation with C=N plane vibration) 720-875 C-H (Def) (Aromatic nucleus) 500-780 C-X(Str.), -Cl, -I |
| C2 | 26 | 212 | 4.76(4.77) | 27.73(27.76) | frl | 414 | 7.20-7.28 | |
| C3 | 19 | 230 | 4.09(4.12) | 37.42(37.46) | r’n | 417 | 7.10-7.25 | |
| C4 | 21 | 210 | 4.92(4.94) | 22.40(22.43) | r’bc | 412 | 6.90-7.21 | |
| C5 | 22 | 214 | 4.78(4.81) | 21.76(21.82) | br’’ | 413 | 6.30-7.19 | |
|
Series II (X= H, Cl, I, Me, OMe; Y= NO2) |
||||||||
| C6 | 41 | 210 | 6.99(7.03) | 21.19(21.27) | b’rsc | 430 | 6.98-8.40 | |
| C7 | 44 | 220 | 6.62(6.64) | 25.67(25.69) | drs’’nc | 433.6 | 7015-8.35 | |
| C8 | 48 | 248 | 5.78(5.80) | 35.10(35.13) | grn | 436.6 | 7.03-8.32 | |
| C9 | 23 | 242 | 6.83(6.87) | 20.74(20.78) | grc | 431 | 6.92-8.34 | |
| C10 | 26 | 220 | 6.67(6.69) | 20.24(20.25) | drgl | 438.8 | 6.28-8.30 | |
|
Series III (X= H, Cl, I, Me, OMe; Y= Me) |
||||||||
| C11 | 27 | 202 | 4.91(4.92) | 22.39(22.43) | tc’c | 412 | 7.02-7.17 | |
| C12 | 28 | 210 | 4.62(4.65) | 27.08(27.12) | b’rp | 415.8 | 7.15-7.23 | |
| C13 | 29 | 220 | 3.99(4.04) | 36.08(36.70) | bs’’’l | 418 | 7.14-7.21 | |
| C14 | 25 | 210 | 4.79(4.82) | 21.88(21.89) | l’bs’l | 415 | 6.94-7.20 | |
| C15 | 23 | 214 | 6.68(4.69) | 21.27(21.30) | l’bs’l | 416.8 | 6.25-7.19 | |
Abbreviations: b- brown,b’- brick, c- crystal, c’- coloured, d- dark, f- faint, g- glazing, l- leaflets, l’- light, n- needles, p- plate, r- red, r’- reddish, r’’- reflux, s- sandy, s’- shining, s’’- scintillating, s’’’- stout, t- tea, y’- yellowish.
Results and Discussions
Scrutiny of the electronic spectra of fifteen newly synthesized CCBSBA asycyanine colorants (scheme I ) among themselves, with unsubstituted analogues9, with ethenyl colorants10 and finally with 3-methyl substitution instead of 3-(1-methylethyl)substitution reveals fascinating observations.
The β -4’/3’ substituted phenylbutadienyl asycyanine colorants showed uniform red shifts in the absorption maxima in comparison to their corresponding β – phenyl analogues irrespective of the nature of additional groups attached to phenyl nucleus i.e. whether they are electron attracting viz. NO2 group or electron donating viz. CH3 group. It is also observed that β -3’-nitro derivative show red shifts than that of β -3’-methyl analogues. Therefore β -3’-differently substituted phenyl group or β-phenyl group itself attached with the methine chain of the butalogues, both result in very similar red shifts corroborating the previous findings5-7.
Remarkable variations was discernable when the absorption maxima of the chain β-4’-substituted ethenylogues were collated with these synthesized analogues . The absorption maxima of the ethenylogues colorants10 were fairly lower than the synthesized analogues colorants. This was due to less resonance in former with decrease in conjugated chain (i.e. shortage of chain ). The absorption maxima of hexatrienyl analogues colorants6,15 were higher than synthesized β –analogues colorants. This was due to greater resonance in the former with increase in conjugated chain (i.e. chain enhancement ). When electronic characteristics of these colorants having 3’-substituted phenyl system were collated with reported 4’-substituted phenyl analogues11,14 blue shifts were observed. It may be due to the fact that in former inductive effect was applicable where as in later resonance and inductive effect both were effective. In the benzothiazole nucleus the effect of 6-substituents or 3-(1-methylethyl) –substituent is small but uniform and systematic for all the colorants. The successive increase in the mol. Wt. of 6- substituent bring about successive red shifts in all the series.
Acknowledgement
The authors express their gratitude to departmental colleagues for their suggestions and CDRI, Lucknow for spectral data.
References
- Kim, S.H.; Matsuoka, M; Kubo, Y.; Yodoshi, T.; Kitao, T.; Dyes and Pigments (England) 7, 1986, 93; Zollinger, H. in “Colour Chemistry”; VCH, 1991; D-6940, Weinhein,(Germany), XVI.
- Hamer, F.M. in “Cyanine Dyes and Related Compounds”, Inter Science Publishers Inc.; New York 1964;Takaji, K.; Matsuoka, M.; Kubo, Y.; Kitao, T.; Dyes and Pigments, 6, 1985, 75.
- Venkatraman, K. in “Chemistry of Synthetic Dyes” Vol –III, 2009
- Shindy, H.A.; Soleiman H.A.; Canadian j. Chem.Eng.& Tech. Vol-I, No.-4, 2010, 44-59.
- Ansari, A.S.; Jha, D. Kumari and Jha, R. K. Proc. International Pers. In Bio-org-Chem.; 1994 A.P., 63
- Ansari, A.S. and Gupta A.K.; Asian J Chem; 1997, 9(2), 265-271.
- Ansari, A.S.; Jha, D. Kumari; Jha, R.K.; and Jha, B.N.; Indian Counc. Chem. 9, 1995, 21-30.
- Jha, B.N.; Banerji J.C.; Dyes and Pigments 1, 1983, 161; 6, 1985, 213; 7, 1986, 133.
- Jha, B.N.; Jha, R.K.; Jha, D. Kumari; Jha, S.N.; Dyes and Pigments 13, 1990, 135-154.
- Ansari, A.S.; Banerji, J.C.; J. Ind. Chem. Soc. 75, 1998, 108-110.
- Ansari, A.S.; Kumar Arvind; Oriental J. Chem. 15(1), 1999, 149-152.
- Ansari, A.S.; Narayan Bhupendra; Proc. International Conf.on Chem. & 13th ann. Conf. Of Ind. Chem. Soc. 1999, A, PPC-12
- Ansari, A.S.; Narayan, B.; Proc. Ind. Counc. Chem. 2004, 111-112.
- Ansari, A.S.; Kumar Arvind, J Chinese Chem. Soc.; 51, 2004, 561-564.
- Ansari, A.S,; Narayan, B. and kunwar G.K.; J. Chinese Chem. Soc. 53, 2006, 1157-1160.
- Ansari, A.S.; Ali, I.; Haque, M.W.; Khan, M.H.; Proc. 3rd Bihar Vigyan Congress, G1-11, 2010, 6
- Ansari, A.S.; Haque, M.W.; Khan, M.H., Ali, I.; Narayan, B.; Proc. 29th ann. Conf. of Ind. Counc. Chem. OP-32, 2010, 145.

This work is licensed under a Creative Commons Attribution 4.0 International License.









