Synthesis, Structure and Luminescent Properties of Ti(Iii),V(Iii) Transition Metal Polymeric Macrocyclic Complexes Derived from Phenanthroline and Biphenyl Groups
Sunil Kumar and T.R. Sharma
Department of Chemistry, K.G.K. Degree College, Moradabad - 244 001(India).
A fully conjugated ligand 4,42 -bis(1,10-phenanthroline-[5,6-d]imidazole-2-yl)-biphenyl based on 1,10-phenanthroline and biphenyl groups was firstly synthesized. The corresponding polymeric macrocyclic complexes with Ti(III) and V(III) were synthesized and characterized by FT-IR, elemental Analysis, conductivity measurement, UV-Vis and luminescence spectra at room temperature revealed that both the polymeric complexes emit blue luminescence at 453 and 452 nm (?em, max) in DMSO solution and blue/green luminescence at 527 and 536 nm (?em, max) in solid state respectively, and the maximum wavelengths of the polymeric complexes are red shifted, compared with the ligand. On the bases of study performed and discussed an octahedral environment seems to exist around metal ion in the complex
KEYWORDS:Polymeric Macrocyclic Complexes; Luminescence; 1,10-Phenanthroline; Biphenyl
Download this article as:| Copy the following to cite this article: Kumar S, Sharma T. R. Synthesis, Structure and Luminescent Properties of Ti(Iii),V(Iii) Transition Metal Polymeric Macrocyclic Complexes Derived from Phenanthroline and Biphenyl Groups. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Kumar S, Sharma T. R. Synthesis, Structure and Luminescent Properties of Ti(Iii),V(Iii) Transition Metal Polymeric Macrocyclic Complexes Derived from Phenanthroline and Biphenyl Groups. Available from: http://www.orientjchem.org/?p=23502 |
Introduction
Recently, polymeric metal complexes have attracted most researchers interests, (1) because they not only exhibit the properties of polymers, but also possess properties of inorganic and organic small molecule metal complexes, such as good thermal stability, processibility and easy film-forming ability. The polymeric metal complexes are considered as promising light-emitting materials, which have good electron transition and intra-molecular charge transfer transitions properties.(2) 1,10-phenanthroline has a rigid framework and possesses a superb ability to chelate many metal ions via two nitrogen donors, which show potential for technological applications, due to their high charge transfer mobility, bright light-emission and good electro- and photo-active properties.(3,4) In our work, 1,10-phenanthroline and biphenyl are interlinked through imidazol rings to form the ligand. The introduction of biphenyl unit enhances π-conjugation and rigidity of the ligand. The ligand complexes, these polymeric metal complexes emission will possibly be enhanced, because the π-conjugation structure can lead to decrease the loss of energy by thermal vibrational decay and enhance their thermal stability. Thus, we designed and synthesized two new polymeric complexes: 4,4′-bis(1,10-phenanthroline-[5,6-d]imidazole-2-yl)-biphenyl with Ti(II), V(II), and systematically investigated photoluminescent properties of these complexes for the first time. These polymeric complexes are considered to be promising materials for organic electroluminescent diodes (OLEDs), further work on fabricating organic electroluminescent devices is under investigation.
Experimental
Material
All of the chemicals and reagents used were of AR grade or equivalent purity. Titanium(III)chloride was prepared in the lab from 12% solution of Ti(III) chloride (B.D.H) by the reported method. All other metal salts were purchased from the market and used as such.
Instrument and measurements:
The ligand and its complexes were subjected to elemental analyses for C, H, and N at CDRI lucknow, melting point, molar conductance and gravimetric estimation of the metals were carried out in the college lab. Magnetic susceptibility was determined by Gouy’s balance using CuSO4.5H2O as calibrant. Electronic spectra of the complexes were recorded by Beckmann-Du spectrometer. The fluorescence spectra were conducted on a Perkin-Elmer LS55 luminescence spectrometer with xenon lamp as the light source. IR spectra were recorded in KBr phase at CDRI lucknow.
Synthesis of 4,4¢-bis(1,10-phenanthroline-5,6-dioneimidazole-2-yl)-biphenyl
The starting material 1,10 phenanthroline 5,6-dione was prepared by the reported method. Hexamethylenetetramine and 4,4¢,bis(chloromethylbiphenyl) were treated in ethanol to produce 4,4¢,-diformylbiphenyl. 1,10-phenanthroline 5,6,dione was prepared from 1,10-phenanthroline, a mixture of concentrated H2SO4 & concentrated HNO3 followed by NaOH. The desired ligands was prepared from the above two synthesized compound.
A mixture of 1, 10-phenanthroline-5,6-dione (1.261g, 6 M mol), ammonium acetate (9.25 g, 120 M mol), 4, 4¢-diformylbiphenyl (0.63 g, 3 M mol ) and glacial acetic acid ( 100 mL) was added to a three-necked flask and refluxed for 3h, and then cooled to room temperature. The precipitate was collected, washed enough with H2O for three times, and dried in vacuum at 60°C, a greenish, yellow powder was obtained, yield 1.42g (80%), 1HNMR(DMSO, d/ppm) : 13.9 (2H, S, H-Py), 9.1 (4H, d, H-Py), 8.9 (4H, d, H-Py), 8.4 (4H, d, H-Py), 8.1 (4H, d, H-ph), 7.8 (4H, d, H-Ph). IR (KBr, Cm–1), 3412, 1617, 1558, 1427, 1463, 822, 739, 686, 693 Cm–1
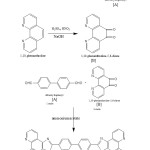 |
Scheme 1 |
Results and Discussion
Synthesis and characterization of Polymeric transition metal complexes
All synthesis routes are shown in scheme 1,10-phenanthroline-5,6-dione were synthesized according to the published literature.(5-11) A methanol solution of metal salt was added to a chloroform solution of ligand. The reaction mixture was stirred for 24h at room temperature, then the solvent was evaporated under reduced pressure, the residue was filtrated and dried.
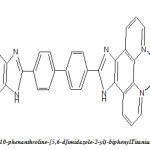 |
Table 1: Click here to View scheme |
The 1HMR spectrum of the ligand in DMSO shows proton signals at d 9.1, 8.9, 8.4, 8.1and 7.8 ppm can be easily assigned to each of the corresponding hydrogen respectively, which indicates the presence of the phenanthroline and benzene rings. The proton signal at d 13.9 ppm corresponds to H – N proton of the imidazole rings. In the IR spectrum of the ligand the absorption peak of N–H is observed at 3412 cm–1, the absorption peak at 1617 and 1558 cm–1 are assigned to N=C and C=C bond stretching frequency, respectively. All these result prove that the ligand has been successfully synthesized.
By comparison with the ligand, the FT-IR spectra of polymeric metal complexes are found to have similar bands. The C=N band in the polymeric complexes shifts to 1628 and 1630 cm–1, the C=C band shifts to 1401 and 1402 cm–1, respectively. Other bands in the complexes have some different extent of shifting, which are similar to the description given in the literature.(12-14) The shift of all bands of the polymeric complexes is attributed to the fact that the ligand was coordinated with the metal ions. At the same time, some new sharp bands are observed at 554 cm–1 and 548 cm–1, which assigned to the ν (M–N) stretching vibration, respectively.(15,16)
In addition, the molar conductance values of the polymeric complexes in DMSO solutions (10–4 M) are 25 and 16 cm2 Ω–1 mol–1, which are lower than the reported range for electrolytes in DMSO solutions.(17) There are weak electrovalent bond between chlorine ions and metal ions, these results are consistent with the light-electrolytes (1:1) nature of the complexes. These data indicate that the ligand successfully chelated to metal ions.
Optical properties of the ligand and the polymeric complexes
| sr. | Ligand and complexes | Luminescence spectra | ||||
| Absorption (nm) | In DMSO (10–4 M) | In solid state | ||||
| λmax (em) | λmax (ex) | λmax (em) | λmax (ex) | |||
| 1. |
4, 4’-bis(1, 10-phenanthroline-[5,6-d]imidazole-2-yl)-biphenyl |
284,322,374 | 438 | 343 | 507 | 411 |
| 2. |
4, 4’-bis(1, 10-phenanthroline-[5,6-d]imidazole-2-yl)-biphenylTitanium(III)chloride
|
308,365 | 448 | 389 | 524 | 458 |
| 3. |
4, 4’-bis(1, 10-phenanthroline-[5,6-d]imidazole-2-yl)-biphenylVanadium(III)chloride
|
298,363 | 457 | 379 | 539 | 432 |
In the solid state, the ligand emits very weak luminescence, which can be assigned to π–π* transition.(18) However, the polymeric complexes emit intensive blue/green light, implying the energy transfer process from metal to ligand take place. The introduction of metal ions enhances the conformational rigidity in the molecule structure and reduces the non-radiative decay of the MLCT excited state, so the polymeric complexes are apt to emit fluorescence.
From the result of TGA, the polymeric complexes are found to have formed stable five-member chelated ring,(19,20) which may be attributed to the fact that the M–N bond is highly polarized.(21) All the complexes possess a very high transition temperature, which are critical for electroluminescence device stability and good lifetime.(22)
Conclusion
Two new polymeric macrocyclic complexes were successfully synthesized and characterized. On the basis of the evidence discussed above an octahedral environment may be suggested around every metal ion in this polymeric complex. The fluorescent spectra of complexes displayed blue/green luminescence at 527 and 536 nm (λem, max) in solid state and blue luminescence at 453 and 452 nm (λem, max) in DMSO solution, respectively. The polymeric complexes have good thermal stability with initial decompose temperature at above 250°C. We expect that the two polymeric complexes can be used as organic electroluminescent diodes (OLEDs).
References
- Xie J, Fan L, Su J and Tian H 2003 Dyes and Pigments 59 153-162
- Zhong C, Guo R, Wu Q and Zhang H 2007 React. Funct. Polym. 67 408
- Felder D, Nierengarten J F, Barigelletti F, Ventura B and Armaroli N 2001 J. Am. Chem. Soc. 123 6291
- Liu Q D, Jia W L and Wang S N 2005 Inorg. Chem. 44 1332
- Yamada M, Tanaka Y, Yoshimato Y, Kuroda S and Shimao I 1992 Bull. Chem. Soc. Jpn 65 1006
- S. Balasubramanian, Inorg. Chem. 26 (1987) 553.
- S. Balasubramanian , Journal of Luminescence 106 (2004) 69–76
- Sahin E, I`de S, Kurt M and Yurdakul S 2002 J. Mol. Struct. 616 259
- Yurdakul S and Kurt M 2003 J. Mol. Struct. 650 181
- Ramesh V, Umasundari P and Das K K 1998 Spectrochim. Acta. A. 54 285
- Dolaz M, Tumer M and Digrak M 2004 Trans. Met. Chem. 29 528
- Geary W 1971 Coord. Chem. Rev. 7 81
- Perkovic M W 2000 Inorg. Chem. 39 4962
- Xia C K, Lu C Z, Zhang Q Z, He X, Zhang J J and Wu D M 2005 Cryst. Growth. Des. 5 1569
- Yutaka T, Obara S, Ogawa S, Nozaki K, Ikeda N and Ohno T 2005 Inorg. Chem. 44 4737
- Zhao W, Zhu H F, Okamura T-A, Sun W Y and Ueyama N 2003 Supramol. Chem. 345
- Yue S M, Xu H B, Ma J F, Su Z M, Kan Y H and Zhang H J 2006 Polyhedron 25 635
- El-Dissouky A 1987 Spectrochim. Acta Part. A 43 1177
- El-Dissouky A, Hendawy A M and Abdel S A 1986 Inorg. Chim. Acta 119 207
- Gad AM, El-Dissouky A, Mansour E M and El-Maghraby A 2000 Polym. Degrad. Stab. 68 153
- Wu Q, Esteghamatian M, Hu N X, Popovic Z D, Enright G and Breeze S R 2001 Chem. Mater. 2079
- Tokito S, Tanaka H, Noda K, Okada A and Taga Y 1997 Appl. Phys. Lett. 70 1929

This work is licensed under a Creative Commons Attribution 4.0 International License.









