Synthesis of Silver Nanoparticles and their Antiproliferation against Human Lung Cancer Cells In vitro
Guoqiang Zhou* and Wenying Wang
Chemical Biology Key Laboratory of Hebei Province, College of Chemistry and Environmental Science, Hebei University, Baoding - 071 002, (China).
A simple and inexpensive, single step synthesis of silver nanoparticles was achieved using sodium citrate both as a reducing and capping agent. Silver nanoparticles were synthesized by injecting sodium citrate solution into the solution of silver nitrate at high temperature. The synthetic process was carried out in aqueous solution, making the method versatile and ecofriendly. The synthesized silver nanoparticles were characterized by UV-visible absorption spectroscopy, field emission scanning electron microscopy (FE-SEM), and dynamic light scattering (DLS). Results showed that the average size of spherical silver nanoparticles was about 30 nm. The antiproliferation activities of the silver nanoparticles on human lung cancer cells were also studied in vitro.
KEYWORDS:Silver Nanoparticles; Synthesis; Antiproliferation; Cytotoxicity
Download this article as:| Copy the following to cite this article: Zhou G, Wang W. Synthesis of Silver Nanoparticles and their Antiproliferation against Human Lung Cancer Cells In vitro. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Zhou G, Wang W. Synthesis of Silver Nanoparticles and their Antiproliferation against Human Lung Cancer Cells In vitro. Available from: http://www.orientjchem.org/?p=11825 |
Introduction
Metal nanoparticles have wide range of applications in various areas of physics, chemistry, material science, and biological science. Intrinsic chemical optical, electronic, sensing, and catalytic properties of metal nanoparticles largely depend on their shapes and sizes1. Silver is a naturally occurring precious metal, most often as a mineral ore in association with other elements. It has been positioned as the 47th element in the periodic table, having a atomic weight of 107.8 and two natural isotopes 106.90 Ag and 108.90 Ag with abundance 52 and 48%. Silver nanoparticles (Ag NPs) have been the focus of great interest because of their unique optical properties such as their applications to ink-jet printing, cosmetics, ammonia sensing, household appliances, antibacterial performance, and so on2. Monovalent silver compounds have been used extensively for antimicrobial treatment and prophylaxis for decades, and recent studies suggest that the antimicrobial activity is retained in Ag NPs3,4. There is also growing evidence that as well as being toxic to bacteria, Ag NPs are also high toxic to brain and stem cells5,6.
There are several reported methods for the preparation of Ag NPs such as citric acid reduction, electrochemical synthesis, photochemistry, and radiation reduction7. But nowadays, a molecule which can act both as the reducing and capping agent are preferred, since in this case the reaction takes place in one step and there is no need for an external reducing agent. This may enable greater control over the reaction parameters and also condense the number of steps involved in synthesis of the metal nanoparticles8. In this work, Ag NPs were synthesized in aqueous medium. The effects of reaction time and concentrations of reducing agent were studied in detail. The Ag NPs were characterized by UV-Vis spectroscopy, dynamic light scattering (DLS), and Field emission scanning electron microscopy (FE-SEM). The antiproliferation effects of Ag NPs on human pulmonary adenocarcinoma cells A549 were investigated. The results indicated that the Ag NPs inhibited the proliferation of A549 cells following dose and time dependent manner. Our results signified that Ag NPs synthesized by chemical reduction method are suitable for formulation of new types of anticancer materials.
Experimental
Materials and reagents
All the chemicals used in this investigation were analytical grade materials and used without further purification. Sodium citrate (C6H5Na3O7·2H2O, ≥ 99%), and silver nitrate (AgNO3, 99%) were purchased from Aldrich Chemical Co. Human pulmonary adenocarcinoma cells A549 were purchased from the ATCC (Beijing, Zhongyuan LTD, China). Dulbecco,s modified Eagle,s medium (DMEM) and trypsin were puchased from GIBCO, USA. New born calf serum was from Hangzhou Sijiqing Organism Engineering Institute. MTT (3-(4,5-Dimethylthi-azol-2-yl)-2,5- diphenyltetrazolium bromide), dimethylsulfoxide(DMSO), penicillin, streptomycin were from Sigma Aldrich. Deionized water was used to prepare aqueous solutions.
Synthesis of silver nanoparticles
The synthesis of colloidal silver nanoparticles (Ag NPs) involved a simple aqueous phase mixing of AgNO3 to varying concentration of C6H5Na3O7·2H2O solution. For the preparation of Ag NPs, a solution of AgNO3 (1.0 × 10-3 M) in deionized water was heated until it began to boil. Sodium citrate solution was added dropwise to the AgNO3 solution as soon as the boiling commenced. The color of the solution slowly turned into grayish yellow, indicating the reduction of the Ag+ ions. Heating was continued for an additional period of time, and then the solution was cooled to room temperature before employing for further experimentation.
Characterization
The nanoparticle formation was monitored by the UV-vis spectra using a UV-vis spectrophotometer system (Agilent 8453 Spectrophotometer). UV-vis study was done using a quartz cell of thickness of 1cm in the wavelength range (200–1000) nm. The progress of reaction was accompanied by the color change of the reaction mixture within the glass bottle. The morphology and size of synthesized Ag NPs were measured by field emission scanning electron microscope (JSM-7500F, JEOL, Japan). A minute drop of nanoparticles solution was cast on to a carbon-coated copper grid and subsequently drying in air before transferring it to the microscope. The size distribution of the nanoparticles in medium was evaluated by dynamic light scattering (Delsa Nano C, Beckman, USA). Data were analyzed based on six replicated tests.
Cell culture
Human lung alveolar carcinoma epithelial cells (A549) were cultured in DMEM under a humidified atmosphere (5% CO2 plus 95% air) at 37 ℃. All media were supplemented with 10% heat inactivated new born calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Cell proliferation assay
A549 Cells (2 × 103 cells/100 μl) were seeded onto 96-well plates and incubated overnight at 37 ℃ under a 5% CO2 atmosphere. The medium in the wells was then replaced with fresh medium containing nanoparticles (2.5-20 μg/ml) and incubation continued for 24 or 48 h. The effects of the nanoparticles on cell viability were determined using the MTT assay. Briefly, 10 μl of MTT solution was added to each well and the plates incubated for 4 h. The supernatant was removed and DMSO (100 μl) was added to solubilize the MTT. The absorbance at 570 nm of each well was measured with a microplate spectrophotometer (BioRad Model 3550, USA). Cells incubated without nanoparticles were used as a control. The cell viability was calculated according to the formula: Asample / Acontrol × 100%.
Results and Discussion
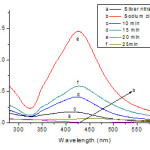 |
Figure 1: UV-Vis absorbance spectra of the Ag colloids at 420 nm at different reaction boiling times. |
During the addition of the C6H5Na3O7·2H2O to the aqueous AgNO3 solution, a light yellow colour slowly appeared in the mixture, indicating the formation of Ag NPs. The intensity and colour variations between the samples arise from the reaction times. The characterization absorption spectra was the important properties of the Ag NPs, and the UV-Vis spectra was a good method for characterization of the formation and growth of Ag NPs. The variation may be attributed to the number of particles and the size distribution in the solution. These differences were more evident when looking at the UV-Vis spectra of the samples as represented in Figure 1. AgNO3 and C6H5Na3O7·2H2O solution had no absorption peaks in 300 ~ 600 nm wavelength range. The UV-Vis absorption band centered at 420 nm was the characteristic absorption of spherical Ag NPs. The maxima absorption peak presented at 420 nm of the synthetic products, which confirmed the samples were Ag NPs. When the size of
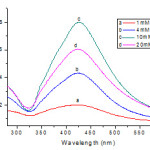 |
Figure 2: UV-Vis absorption spectra of Ag colloids prepared using different concentration of sodium citrate as the reductant. |
the particles was small, the absorption band was broad and the peak intensity was low. By increasing the size, the absorption spectrum became narrower with an increase in the intensity of the absorption band and a decrease in the bandwidth. The reaction time for citrate reduction of Ag ions at boiling temperature was found to be important for achieving complete reduction. For example, if we quickly cool the solution after boiling for 5~15 min, we obtain only partial reduction. With the extension of reaction time, a red shift phenomenon was observed and the absorption peak intensity first increased then decreased. The size distribution in aqueous medium was investigated using a DLS method. When the reaction time was 20 min and the concentration of sodium citrate was 10 mM, the size distribution in aqueous medium was narrowest and the average size of spherical Ag NPswas 30.2 ± 2.6 nm (Fig.3(A)). So the optimum reaction time for the synthesis of Ag NPs was 20 min.
Effects of concentrations of citrate on synthesis of silver nanoparticles
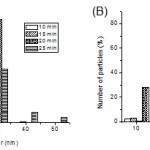 |
Figure 3: Size distribution of Ag nanoparticles in medium as measured by dynamic light scattering (DLS). (A) At different reaction time; (B) At different concentrations of C6H5Na3O7·2H2O. |
The absorption spectra of the silver colloids obtained using different concentrations of citrate and boiling for 20 min are shown in Figure 2. The observed increase in the plasmon absorbance at 420 nm with increasing citrate concentration indicates a greater amount of Ag+ reduction. These observations showed that the growth of Ag NPs was influenced by the concentration of C6H5Na3O7·2H2O. The decrease seen in the maximum absorbance in trace a as compared to trace c in Figure 2 shows that a small fraction of the Ag+ is likely to remain unreduced at low citrate concentrations. Whereas most of the reduction is completed at a higher citrate concentration. The absorbance maximum at 420 nm against concentration of citrateshows that the absorbance increases with increasing citrate concentration and attains a plateau at higher concentration of citrate ions (Fig 2). Obviously, at these high concentrations, we achieve complete complexation of the Ag2+ species with citrate ions. Henglein et al9 in a recent study of citrate capping of silver clusters have pointed out that at low concentrations of citrate radiolytic reduction produces well separated small spherical particles. On the other hand, at higher citrate concentration, they observed large-sized clusters with broad size distribution. They attributed this observation to the destabilization effect of citrate as it induces coalescence. The optimum concentrations of C6H5Na3O7·2H2O for the synthesis of Ag NPs was 10 mM.
SEM investigations
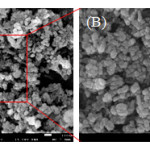 |
Figure 4: Field emission scanning electron microscope (FE-SEM) images with different magnifications (A-B) of the spherical Ag nanoparticles. |
Field emission scanning electron microscopy (FE-SEM) was used to investigate the morphology and size of the synthesized Ag NPs. The Ag NPs obtained with the sodium citrate reduction method were predominantly spherical with diameters ranging from a few nanometers to well above 30 nm. Fig. 4 (B) is the local magnified panel of Fig.4 (A) and shows the typical images of the spherical samples.
Effects of silver nanoparticles on proliferation of cell lines
Human lung alveolar carcinoma epithelial cells A549 was continuously treated with Ag NPs for 24 or 48 h at concentration range from 2.5 to 20 μg/ml. Ag NPs inhibited the proliferation of A549 cells following dose and time dependent manner. For the total range of concentrations, the most pronounced inhibition was found at 48 h (p < 0.01). As shown in Fig 5, Ag NPs inhibited the proliferation of A549 cells significantly at concentrations of 5, 10 and 20 μg/ml for 48 h. After A549 cells were exposed to Ag NPs at 5, 10 and 20 μg/ml for 48 h, cell viability of the groups exposed to Ag NPs decreased to 87.1%, 75.2% and 68.5%, respectively, compared to the control group.
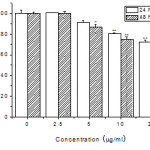 |
Figure 5: Effects of Ag nanoparticles on the proliferation of A549 cells. (*P < 0.05, **P < 0.01 compared with the control group, n = 6). |
In conclusion, spherical shaped AgNPs were synthesized successfully by chemical reduction of AgNO3, which is a template-less and seed-less method. UV-Vis spectra and DLS results showed that concentration of the reducing agent and reaction time had significant effects on the formation and size distribution of AgNPs. We studied the optimum conditions in which the stabilized AgNPs were created. This method can be further extended to prepare other noble metal nanoparticles of various morphologies. AgNPs inhibited the proliferation of A549 cells following dose and time dependent manner. These results demonstrated AgNPs prepared by the chemical reduction method described here have great promise as antitumor agents. Application of AgNPs based on these findings may lead to valuable discoveries in various fields such as medical agents.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21001038).
References
- Li C. Z., Male K. B., Hrapovic S., Chem. Commun., 31,3924 (2005).
- Shim I. K., Lee Y. I., Lee K. J., Joung J., Mater. Chem. Phys., 110,316 (2008).
- Ip M., Lui S. L., Poon V. K. M., Lung I., J. Med. Microbiol., 55,59(2006).
- Melaiye A., Sun Z., Hindi K., Milsted A., J. Am. Chem. Soc., 127, 2285(2005).
- Hussain S. M., Javorina A. K., Schrand A. M., Toxicol. Sci., 92,456(2006).
- Braydich L. S., Hussain S., Schlager J. J., Toxicol. Sci., 88,412(2005).
- Song W. H., Zhang X. X., Yin H. Z., Chin. J. Chem., 27, 717(2009).
- Si S., Mandal T. K., Chem. Eur. J., 13, 3160(2007).
- Henglein A., Giersig M., J. Phys. Chem. B., 103, 9533(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









