Synthesis of Novel Aryl Quinoxaline Derivatives by New Catalytic Methods
Reza Soleymani1 *, Neda Niakan2 , Shohre Tayeb2 and Shirin Hakimi3
1Young Researchers Club, Shahre-Rey Branch, Islamic Azad University, Tehran (Iran). 2Department of Chemistry, Shahre-rey Branch, Islamic Azad University, Tehran (Iran). 3Faculty of Science, Department of Chemistry, Khajeh-Nasir
Quinoxalines are nitrogenous compounds that widely are used in various industries like paint, pharmaceutical and medicine. Quinoxaline ring is also part of antibiotics structure such as Actinomycin, Lomacin. By using of this synthesis method of these compounds based on condensation of Aryl-1,2-di amine with 1,2-di carbonyl in the Acidic condition, some new compounds was obtained from Quinoxaline family that for the first time some new catalysts was used for increasing of efficiency and reducing of process time. Used catalysts are CrCl2.6H2 O, PbBr2 and CuSO4.5H2O compounds that preparation of these catalysts is economically, cost-effective and saves time. All mechanisms were done in ethanol solvent at room temperature. However, all results suggest a mild and heterogeneous nature of these mechanisms, shorter time of reaction and higher yield or similar to reported old methods.
KEYWORDS:IR; NMR; Quinoxalines; Synthesis; New Catalytic methods
Download this article as:| Copy the following to cite this article: Soleymani R, Niakan N, Tayeb S, Hakimi S. Synthesis of Novel Aryl Quinoxaline Derivatives by New Catalytic Methods. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Soleymani R, Niakan N, Tayeb S, Hakimi S. Synthesis of Novel Aryl Quinoxaline Derivatives by New Catalytic Methods. Available from: http://www.orientjchem.org/?p=11837 |
Introduction
Hetero cycles compounds are used in many various industries. However most of hetero cycles compounds aren’t extracted from nature source, but are synthesized. Almost all alkaloids that are used as drugs are formed from hetero aromatic molecules. Because these compounds cause to cancer, these chemicals must be removed from output materials of smokestack in factories [1,2]. Some of these compounds are used as pigment and some additive in food industry and sometimes are applicable in pharmaceutical industry [3]. Quinoxalines are a family of hetero aromatic ring. Warfarin is derived from Coumarin that is anti-coagulation. Prazosin compound is belong to the anti-hypertensive that is Quinazoline family. Quinine structure which is known as a anti malaria drug [4-6]. However, Quinoxalines and derivatives which are called Benzopyrazine is the most important compound of Nitrogen Heteroaromatic. The combination of component family Benzo-Heterocycles to the floor and classified as very important intermediate compounds in organic and inorganic chemistry [7]. Although these compounds are rarely found in nature, but many Quinoxaline derivatives are known in the pharmaceutical industry and wide area of biological activities including anti-virus, anti-diabetic, anti-HIV, anti-fungal, anti parasitic, Anti-cancer, anti-bacterial and anti-depression has been reported in the literature for this compound [8-11]. Pyrazine amide (anti-TB drug) and Merfazin amide are prepared from Quinoxalines during several steps [12]. In this synthesis, Biscarboxylic is produced by oxidation of Quinoxaline and after that Pyrazine amid is obtained by easy decarboxylation the oxidation products are Biscarboxylic, then by removing of ,Esterification, Aminolize and by Mannich reaction, Merfazin amid is produced. Quinoxaline ring is part of the structure of some anti-biotics such as Actinomycin, Lomacin and Actinolite that is known as a preventer agent of growing of Gram-positive bacteria and active compound against different migratory tumor [13]. However, application of Lomacin has been reported in dental [14]. Some Quinoxalines with high electron donor properties have been used in polymer chemistry with conjugated π systems [15]. However, it’s known that oxidation of Nitrogen atoms of Quinoxaline ring significantly caused to increasing of the biological activity of this compound. Quinoxaline combination o4 1,4-di–N-oxide inhibits TB progression with 99 to 100% efficacy [16]. Quinoxalines also have been used as a pigment in the technology of organic dyes [17]. Some of the color-causing substances are reactive dyes that show good stability in cold alkaline solution and have high reactivity such red Di-Chloro Quinoxalin [18]. But in this industry Quinoxalin has been used as a catalyst and agent of reducing azo dyes. These catalysts react with the silver and cationic radicals are produced which are in the forms of Hetero-Aromatic and Di-Hydro-Hetero Aromatic. In some references, the Quinoxaline is mentioned as building blocks in the synthesis of anion recipient, DNA fragmentation and the chemical switch control [19-23]. In other cases it can be pointed to the use of Quinoxaline in effective electroluminescence materials [24]. There are different ways to synthesize of Quinoxaline such as density ortho-Phenylenediamine with Glyoxal Sodium Bisulfite [25], Hinsberg reaction [26], coupling the oxidation Epoxides and N-1,2-diamines [27], Cycloaddition α-Aryl Imino Oximes in carbonyl compounds [28,29] coupling Suzuki [30], synthesis of Quinoxalines by Amino Mercuration oxidation Propargyl alcohol [31] and also there are various method for synthesis of these compounds. In this synthetic method of these compounds is used based on accumulation of Aril 1,2-di amin with 1,2-di carbonyl in Acidic conditions [32-36]. Because this method has advantages such as high efficiency, decreasing of reaction time and availability and saving time and cost than other methods. However, using of different catalysts can be changed the rate and reactivity of these compounds. For this purpose some catalysts like CrCl2.6H2O, PbBr2 and CuSO4.5H2O were used and these variations were studied.
Experimental Details
General method
All materials in these experiments were obtained from Merck, Germany. All melting points and efficiencies have been reported related to the pure products. Synthetic compounds were compared with spectroscopic data and physical properties of real samples. Progress of reactions was with thin layer chromatography (TLC) at first till completion of reaction. Used TLC plates are G/UV 254 nm-TLC-Grade silica gel. Infrared spectrums of the precursor and final products were recorded by IR-470 infrared spectrophotometer equipped with Shimatzu companies’ monitor. 1H-NMR and 13C-NMR spectra of final products were recorded by NMR 400 MHz broker, Avance 2 model. For purifying, especially in the vacuum distillation, solvent removal and drying of products were used from a vacuum pump model OV-42N-250 from U.S.A. for determination of melting point, the sample is powdered in a porcelain mortar, and then the melting point was measured by the UK company British Barnstead Electro thermal 9100 BZ .
Synthesis
Required amount of Lewis acid and 5 mL ethanol were added to mixture of derivatives of ortho-phenylen di amine (1.10 mol), aromatic 1,2-di ketone (1.00 mmol) as solvent to 25 mL flask equipped to magnetic stirrer in room temperature. All organics were dissolved in a solvent in the beginning of reaction. Progress of reaction was considered by using thin layer chromatography (TLC) in mixture of n-hexane and ethyl acetate. At the end, the mixture of reaction was heated till it was dissolved in hot ethanol and catalysts that wasn’t soluble in ethanol, was separated by simple filtration. After cooling of solution, single crude product was obtained. Synthetic method of three compounds with different catalysts is described as following:
Synthesis method of acenaphtho[1,2-b]quinoxaline by CrCl2.6H2O catalyst
A mixture of ortho-phenylen di amine 0.119 g (1.11 mmol), Acenaphthoquinone 0.183 g (1.01 mmol), proposed catalyst 0.01 g and ethanol 5 mL as solvent were added in flask 25 mL equipped to magnetic stirrer in room temperature. Progress of reaction was considered by using thin layer chromatography (n-hexane and ethyl acetate 2:10). After 14 minutes, reaction was completed. After that the mixture was heated till the product was dissolved in hot ethanol and catalyst was separated by filtration. Cooling of solution, 0.225 g (90%) of milk-white crystal was obtained that melting point were 237 oC;
A milk-white solid; Mp: 237-238 oC; IR (KBr Cm-1): 3060, 1620, 1435, 1307, 835, 768; 13C-NMR (CDC13, 400 MHz) δ(ppm): 155.22, 142.49, 137.72, 132.86, 131.33, 130.97, 130.22, 130.01, 129.58, 122.92; 1H-NMR (CDC13, 400 MHz) δ(ppm): 8.19 (d, 2H, J = 6.8 Hz), 8.08 (dd, 2H, J1= 6.3, J2= 3.5 Hz), 7.85 (d, 2H, J = 8.5 Hz), 7.68 (t, 2H, J = 8 Hz), 7.64 (dd, 2H, J1 = 6.3, J2= 3.5 Hz).
Synthesis method of 6-methyl-[2,3]-di phenyl quinoxaline by PbBr2 catalyst
A mixture of 4-methyl ortho-phenyl di amine 0.134 g (1.10 mmol), Benzyl 0.21 g (1.00 mmol) , proposed catalyst 0.01 gr and ethanol 5 mL as solvent were added in flask 25 mL equipped to magnetic stirrer in room temperature. Progress of reaction was considered by using thin layer chromatography (n-hexane and ethyl acetate 2:10). After 38 minutes, reaction was completed. That the mixture was heated till the product was dissolved in hot ethanol and catalyst was separated by filtration. After cooling of solution, 0.268 g (93%) of milk-white crystal was obtained that melting point were 113 oC;
A milk-white solid; Mp: 113-114 oC; IR (KBr Cm-1): 3045, 2940, 1633, 1350, 765,687; 13C-NMR (CDC13, 400 MHz) δ(ppm): 154.33, 153.65, 142.52, 141.53, 140.77, 140.36, 133.25, 131.04, 129.75, 129.64, 129.45, 129.20, 23.14. 1H-NMR (CDC13, 400 MHz) δ(ppm): 7.84 (d, 1H, J = 8.3 Hz), 7.66 (s, 1H), 7.33 (m, 5H), 7.18 (m, 6H), 2.26 (s, 3H).
Synthesis method of di benzo [a,c] phenazine by CuSO4.5H2O catalyst
A mixture of ortho-phenyl di amine 0.119 g (1.11 mmol), Phenanthrenequinone 0.210 g (1.01 mmol), proposed catalyst 0.01 g and ethanol 5 mL as solvent were added in flask 25mL equipped to magnetic stirrer in room temperature. Progress of reaction was considered by using thin layer chromatography (n-hexane and ethyl acetate 2:10). After 36 minutes, reaction was completed. After that the mixture was heated till the product was dissolved in hot ethanol and catalyst was separated by filtration. Cooling of solution, 0.255 g (92%) of milky-yellowish crystal was obtained that melting point were 229 oC;
A milky-yellowish solid; Mp: 229-230 oC; IR (KBr Cm-1): 3060, 1612, 1485, 1366, 775, 730; 13C-NMR (CDC13, 400 MHz) δ(ppm): 143.44, 143.17, 133.11, 131.34, 130.83, 130.49, 129.06, 127.34, 124.10; 1H-NMR (CDC13, 400 MHz) δ(ppm): 9.15 (d, 2H, J = 7.4 Hz), 8.29 (dd, 2H, J = 7.9 Hz), 8.09 (dd, 2H, J = 6.5, 3.8 Hz), 7.48 (m, 6H).
Synthesis Quinoxaline derivatives by Lewis acids CrCl2.6H2O, PbBr2 andCuSO4.5H2O compounds
Ortho-phenylene di amine derivatives, aromatics α-dicarbonyl and Lewis acid catalysts CrCl2.6H2O, PbBr2 and CuSO4.5H2O in solution phase at the room temperature was used for synthesis of these compounds (Figure 1). For this purpose, catalysts optimizing was not done and a size of all catalysts (0.01 g) was used. Optimizing ratio of raw materials ortho-phenylene di amine and aromatics α-dicarbonyl is 1:1.1. Because of reaction between di amine and Lewis acid catalyst, using of more di amine is necessary.
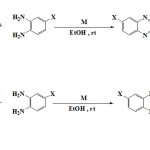 |
Figure 1: Synthetic route for the Quinoxaline derivatives. |
Result and Discussion
Calculation of obtained efficiency values, reaction time and melting point for each compound are reported in tables 1 to 3. The results of table 1 was obtained when CrCl2.6H2O catalyst was used and the result of table 2 was obtained when PbBr2 catalyst was used. Table 3 was obtained when CuSO4.5H2O catalyst was used. As the results shown, by changing of catalyst, reaction time and efficiency was changed.
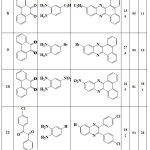 |
Table 1: Yields and reaction conditions of the synthesized quinoxaline derivatives that catalyzed by CrCl2.6H2O compound. |
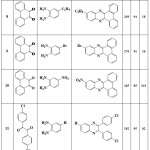 |
Table 2: Yields and reaction conditions of the synthesized quinoxaline derivatives that catalyzed by PbBr2 compound. |
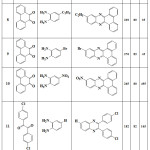 |
Table 3: Yields and reaction conditions of the synthesized quinoxaline derivatives that catalyzed by CuSO4.5H2O compound. |
The proposed mechanism had shown in figure 2. In all cases, ethanol was used as a solvent. In according to catalyst type, because of empty orbital was acted like a Lewis acid and activated carbonyl part of aromatic 1,2-di ketones. At this time, amine group because of non-bonding electron pair on nitrogen atom was done nucleophilic attack to carbon atom joint to oxygen.
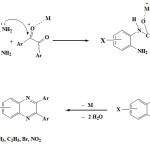 |
Figure 2: The proposed mechanism for synthesis different Quinoxaline derivatives. |
This mechanism is repeated until the removal of two water molecules, Quinoxaline was obtained. Obviously, when there is electron-withdrawing groups such as nitro and chlorine on carbon number 4 of aryl 1,2-diamine ring, because of being positive of reaction center namely nitrogen caused to reduction of reaction progress rate . On the other hand, electron donating groups like methyl on carbon atom number 4 of aryl 1,2-diamines because of hyper conjugation effect of methyl group accelerated reaction. But because the methyl group relative to amine is in para position and relative to the other amines is in the meta position, therefore these two effects offset each other and significant difference in reaction rate compared with non-substituted aryl 1,2-diamine was not observed, see tables 1 to 3.
According to obtained results in the above tables, less reactivity of benzyl compound in comparision with other compounds is considerable that is because of trans formation of this molecule that was required initial energy for reaching to cis formation until nucleophiles attack was provided. Formation of two other di carbonyl was cis and required any initial energy and finally enough energy for reaching to less transition state and reaction rate was increased. This reaction was done in two steps, respectively nucleophilic attack and removal of water that using of large amounts of Lewis acid caused to difficulty passing of the first step of reaction. It’s because of reaction between nucleophile (diamine) and Lewis acid and caused to limitation of nucleophilic attack.
Conclusion
General and basically result was described below:
The reaction in ethanol solvent was done well that is a clean reaction, non-toxic and appropriate with environment. This solvent is inexpensive and doing of this reaction is economical. It can be used for industry, too.
Most reactions was done at room temperature and did not require to temperature conditions. So, the reaction was progressed at mild conditions that was not required to special device for applying of this conditions.
.Reaction time was relatively short and reaction efficiency was relatively high.
The used catalysts against to some reported catalysts in some articles was not required to prior preparation for doing of reaction and are easily accessible with affordable price.
CrCl2.6H2O, PbBr2 and CuSO4.5H2O are very good catalysts for the synthesis of Quinoxaline derivatives. One important feature of these catalysts is insolubility in ethanol that was made heterogeneous system for the reaction and other feature is using few values of catalysts. These catalysts have not annoying odor and are easy to use because of being solid.
Separation of the products was done by a simple filtering.
Acknowledgements
The authors are indebted to Dr Sahar Farsi-madan, missy Maryam Karimi and missy Rezvan Soleymani for their interest in this work and many helpful discussions. However, this work was supported by Islamic Azad University Shahre-rey branch and Khajeh-Nasir Toosi University.
References
- G.M. Badger, The Chemistry of Heterocyclic Compounds, Academic press, New York, (1961).
- A. Joule, G.F. Smith, Heterocyclic Chemistry, Methuen, Van Nostrand-Reinhold, New York, (1972).
- J.M. Clough, C.R.A. Godfrey, Chem. in Britain, 31: 466 (1995).
- L. Krsti, S. Sukdolak, S. Soluji, J. Serb. Chem. Soc, 67: 325 (2002).
- M.L. Bolognesi, R. Budriesi, A. Chiarini, E. Poggesi, A. Leonardi, C. Melchiorr. J. Med. Chem, 41: 4844 (1998).
- S.M. Weinreb, Nature, 411: 429 (2001).
- (a) A.E. Porter, In Comprehensive Heterocyclic Chemistry, 3: 157 (1984). (b) G. Sakata, K. Makino, Y. Kurasama, Heterocycles, 27: 2481 (1988). (c) G.W.H. Cheeseman, E.S.G. Werstiuk, Adv. Heterocycl. Chem, 22: 367 (1978).
- Y.B. Kim, Y.H. Kim, J.Y. Park, Bioorg. Med. Chem. Lett, 14: 541 (2004).
- G. Sakata, K. Makino, Y. Kurasawa, Heterocycles, 27: 2481 (1998).
- M.M. Ali, M.M.F. Ismail, M.S.A. El-Gabby, M.A. Zahran, T.A. Ammar, Molecules, 5: 864 (2000).
- R. Sarges, H.R. Howard, R.C. Browne, L.A. Label, P.A. Seymour, J. Med. Chem, 33: 2240 (1990).
- S. Kushner, H. Dalalian, J.L. Sanjurjo, F.L. Bach, S.R. Safir, V.K. Smit, J.H. Williams, J. Am. Chem. soc, 74: 3617 (1952).
- S. Sato, O. Shiratori, K. Katagiri, J. Antibiot, 20: (1967) 270.
- A. Benagiano, Pro Med, 15: 19 (1963).
- I. L. Finer, J. Chem. Soc, 4: 1205 (1955).
- A. Jaso, B. Zarranz, I. Aldana, A. Monge, Eur. J. Med. Chem, 38: 791 (2003).
- E.D. Brock, D.M. Lewis, T.I. Yousaf, H.H. Harper, The Procter & Gamble Company, USA WO, 9951688 (1999).
- A. Datyner, E. Finnimore, U. Meyer, J. Soc. Dyers Colour, 93: 278 (1997).
- L.S. Jonathan, M. Hiromitsu, M. Toshihisa, M.L. Vincent, F.J. Hiroyuki, Chem. Commun, 8: 862 (2002).
- (a) L. S. Jonathan, M. Hiromitsu, M. Toshihisa, M. L. Vincent, F. Hiroyuki, J. Am. Chem. Soc, 124: 13474 (2002). (b) P.C. Peter, Z. Gang, A.M. Grace, H. Carlos, M.G.T. Linda, Org. Lett, 6: 333 (2004).
- O. Sascha, F. Rudiger, Synlett, 9: 1509 (2004).
- S. Louis, M.G. Marc, J.W. Jory, P.B. Joseph, J. Org .Chem, 68: 4179 (2003).
- M. J. Crossley, L.A. Johnston, Chem. Commun,10: 1122 (2002).
- K.R. Justin Thomas, V.Marappan, T.L. Jiann, C.Chang Hao, T. Yu ai, Chem. Mater, 17: 1860 (2005).
- G.H.C.Woo, J.K. Snyder, Z.K. Wan, Prog. Heterocycl. Chem, 14: 279 (2002).
- (a) O. Hinsberg, Chem. Ber, 23: 2962 (1890). (b) O. Hinsberg, J. Kessler, Chem. Ber, 38: 906 (1905).
- S. Antoniotti, E. Dunach, Tetrahedron Lett, 43: 3971 (2002).
- N.P. Xekoukoulotakis, M.C.P. Hadjiantonious, A.J. Maroulis, Tetrahedron Lett, 41: 10299 (2000).
- Z. Zhao, D.D. Wisnoski, S.E. Wolkenberg, W.H. Leister, Y. Wang, C.W. Lindsley. Tetrahedron Lett, 45: 4873 (2004).
- N. Miyaura, K. Yamada, A. Suzuki, Tetrahedron Letters, 20: 3437 (1979).
- J. Barluenga, F. Aznar, R. Liz, M.P. Cabal, Synthesis, 3: 313 (1985).
- P. Haldar, B. Dutta, J. Guin, J.K. Ray, Tetrahedron Lett, 48: 5855 (2007).
- H. Tai kun, W. Rui, Catal. Commun, 9: 1143 (2008).
- F. da Silva Miranda, A.M. Signori, J. Vicente, B. de Souza, J.P. Priebe, B. Szpoganicz, N. S. Goncalves, A. Neves, Tetrahedron Lett, 64: 5410 (2008).
- L. Yan, F. Liu, G. Day, H. Liu, Bioorg. Med. Chem. Lett, 17: 609 (2007).
- W. Zhu, M. Dai, Y. Xu, X. Qian, Bioorg. Med. Chem, 16: 3255 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









