Synthesis and Investigation of a Novel pH- and Salt-Responsive Superabsorbent Hydrogel Based on Pectin
Mohammad Sadeghi*, Esmat Mohammadinasab and Fatemeh Shafiei
Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak, Iran.
Article Received on :
Article Accepted on :
Article Published : 01 Jun 2012
In the present paper, attention is paid to synthesis and investigates swelling behavior of a superabsorbent hydrogel based on Pectin (Pec) and polyacrylic acid (PAcA). acrylic acid (AcA) was graft copolymerized onto Pectin backbones by a free radical polymerization technique using ammonium persulfate (APS) as initiator and methylene bisacrylamide (MBA) as a crosslinker. A proposed mechanism for hydrogel formation was suggested and the structure of the product was established using FTIR and SEM spectroscopies. Under the optimized conditions concluded, maximum capacity of swelling in distilled water was found to be 348 g/g. Absorbency of the synthesized hydrogels was also measured in NaCl and CaCl2 salt solutions. Results indicated that the swelling ratios in compare to water decreased with an increase in the ionic strength of solution. In addition, swelling capacity was conducted in solutions with pH ranged from 1 to 13. The H-Pecpoly(sodium acrylate) hydrogel exhibited a pH-responsiveness character so that a swellingdeswelling pulsatile behavior was recorded at pHs 3 and 9.
KEYWORDS:Pectin; Hydrogel; pH- and Salt-Responsive; Acrylic Acid Monomer
Download this article as:| Copy the following to cite this article: Sadeghi M, Mohammadinasab E, Shafiei F. Synthesis and Investigation of a Novel pH- and Salt-Responsive Superabsorbent Hydrogel Based on Pectin. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Sadeghi M, Mohammadinasab E, Shafiei F. Synthesis and Investigation of a Novel pH- and Salt-Responsive Superabsorbent Hydrogel Based on Pectin. Available from: http://www.orientjchem.org/?p=11834 |
Introduction
Highly swelling polymers, i.e. superabsorbent hydrogels, are hydrophilic, three dimensional networks that can absorb water in the amount from 10% up to thousands of times their dry weight. They are widely used in various applications such as hygienic, foods, cosmetics, and agriculture [1]. This accounts for increase in the worldwide production of superabsorbent polymers (SAPs) from 6000 tons in 1983 to 450000 tons in 1996 [2-4]. Nowadays, the worldwide production of SAPs is more than one million tons in year. Hence, synthesis and characterization of superabsorbent hydrogels is the main goal of the several research groups in the world(3-6).
Pectin is a naturally occurring biopolymer that is finding increasing applications in the pharmaceutical and biotechnology industry. It has been used successfully for many years in the food and beverage industry as a thickening agent, a gelling agent and a colloidal stabiliser. Pectin also has several unique properties that have enabled it to be used as a matrix for the entrapment and/or delivery of a variety of drugs, proteins and cells.
The modification of natural polymers is a promising method for the preparation of superabsorbt hydrogels. Graft copolymerization of vinyl monomers onto natural polymers is an efficient approach to achieve these materials. Superabsorbing resins were first developed with a view to utilizing agricultural materials, and are typed by the hydrolyzed starch-g-poly(acrylonitrile), H-SPAN[6]. Since then, starches from different resources as well as other polysaccharides, for example, cellulose[8], hydroxyethyl cellulose, agar , sodium alginate and guar gum were graft copolymerized to achieve water absorbing polymers. Polyacrylonitrile (PAN), polyacryamide, and poly(acrylic acid) [10-11] have been frequently grafted, mostly onto starch, using different initiators especially the ceric-saccharide redox system [12]. Radical polymerization, however, has several disadvantages. The reproducibility of this method is poor, and there is little control over the grafting process, so the molecular weight distribution is polydisperse. In addition, the necessity for inert gases (e.g., argon) to prepare an oxygen-free atmosphere and the need for initiators, toxic and/or expensive monomers, and crosslinkers are other disadvantages of free-radical polymerization reactions. These problems have been reviewed in detail [12-13]. For the first time, Fanta et al. [14], with a new method, tried to synthesize of HSPAN superabsorbent hydrogel. They indicated by a solubility test that crosslinks were formed during graft copolymerization, by coupling of the two growing PAN radicals, and during saponification, by the attack of Pectin alkoxide ions on the nitrile groups as the initioation reaction of nitrile polymerization in the early stages of saponification. The nitrile groups of PAN were converted to a mixture of hydrophilic carboxamide and carboxylate groups during alkaline hydrolysis followed by in situ crosslinking of the grafted PAN chains. The initially formed oxygen–carbon bonds between the Pectin hydroxyls and nitrile groups of the PAN chains remained crosslinking sites. Then, Fanta and Doane [14] attempted to extend this idea to the preparation of superabsorbent hydrogels by the saponification of PAN in the presence of polyhydroxy polymers. Finally, Yamaguchi et al.1 [15] reported the preparation of superabsorbing polymers from mixtures of PAN and various saccharides or alcohols. In this investigation, we paid attention to the synthesis and investigation of a superabsorbent based on Pectin and PAcA. The effects of the variables reaction on the swelling properties as well as the salt and pH sensitivity of the hydrogels were investigated.
Experimental
Materials
Pectin (chemical grade, MW 50000) was purchased from Merck Chemical Co. (Germany). Acrylic acid (AcA, Merck) was used after vacuum distillation. Ammonium persulfate (APS, Merck) and Methylene bisacrylamide (MBA, Fluka) were used as received. All other chemicals were of analytical grade.
Preparation of hydrogel
A facial one step preparative method was used for synthesis of Pec-poly(sodium acrylate) hydrogel, Pec-poly(NaAcA), hydrogel. Pectin(0.50-1.5 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 50-500 rpm), including 35 mL doubly distilled water. The reactor was immersed in a thermostated water bath. After complete dissolution of Pectin to form a homogeneous solution , a definite amount of APS solution (0.15 g in 5 mL H2O) was added to pectin solution and was allowed to stir for 10 min. After adding APS, certain amounts of monomer (AcA 1.50 g in 5 mL H2O ) was added to the pectin solution. MBA solution (0.08 g in 5 ml H2O) was added to the reaction mixture after the addition of monomer and the mixture was continuously stirred. After 60 min, the reaction product was allowed to cool to ambient temperature and neutralized to pH 9 by addition of 1N sodium hydroxide solution. The hydrogel was poured to excess non solvent ethanol (200 mL) and kept for 3 h to dewater. Then ethanol was decanted and the product scissored to small pieces. Again, 100 mL fresh ethanol was added and the hydrogel was kept for 24 h. Finally, the filtered hydrogel is dried in oven at 60oC for 10 h. After grinding using mortar, the powdered superabsorbent was stored away from moisture, heat and light[16].
Swelling measurements using tea bag method
The tea bag (i.e. a 100 mesh nylon screen) containing an accurately weighed powdered sample (0.5 ± 0.001 g) with average particle sizes between 40–60 mesh (250-350) was immersed entirely in distilled water (200 mL) or desired salt solution (100 mL) and allowed to soak for 3 h at room temperature. The tea bag was hung up for 15 min in order to remove the excess fluid. The equilibrated swelling (ES) was measured twice using the following equation[17]:
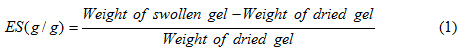
The accuracy of the measurements was ±3%.
Absorbency at various pHs
Individual solutions with acidic and basic pHs were prepared by dilution of NaOH (pH 13.0) and HCl (pH 1.0) solutions to achieve pH≥6.0 and pH<6.0, respectively. The pH values were precisely checked by a pH-meter (Metrohm/620, accuracy ±0.1). Then, 0.5 ± 0.001 g of the dried hydrogel was used for the swelling measurements according to Eq. 1.
pH-sensitivity
pH-sensitivity of the hydrogel was investigated in terms of swelling and deswelling of the final product at two basic (pH 9.0) and acidic (pH 3.0) solutions, respectively. Swelling capacity of the hydrogels at each pH was measured according to Eq. 1 at consecutive time intervals (50 min).
Instrumental analysis
Fourier transform infrared (FTIR) spectra of samples were taken in KBr pellets, using an ABB Bomem MB-100 FTIR spectrophotometer (Quebec, Canada), at room temperature. The surface morphology of the gel was examined using scanning electron microscopy (SEM). After Soxhlet extraction with methanol for 24 h and drying in an oven, superabsorbent powder was coated with a thin layer of gold and imaged in a SEM instrument (Leo, 1455 VP).
Result and Discussion
Synthesis of hydrogels
A general reaction mechanism for Pec-polyacrylic acid hydrogel formation is shown in Scheme 1. At the first step, the thermally dissociating initiator, i.e. APS, is decomposed under heating to produce sulfate anion-radical. Then, the anion-radical abstracts hydrogen from one of the functional group in side chains (i.e. OH) of the substrate to form corresponding radical (alkoxide radicals). Then, these macroalkoxides initiate crosslinking reaction between some adjacent polyacrylic acid pendant chains. In addition, crosslinking reaction was carried out in the presence of a crosslinker, i.e., MBA, so that a three dimensional network was obtained.
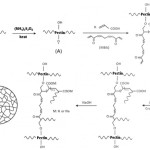 |
Scheme 1 Click here to View scheme |
FTIR spectroscopy
The monomer grafting was confirmed by comparing the FTIR spectra of the pectin substrate with that of the grafted products in figure 1. The bands observed at 1643 cm-1 and 1705 cm-1 can be attributed to C=O stretching in carboxamide and carboxylate functional groups of crosslinker and grafted acrylic acid monomers on to pectin backbone respectively. The band observed at 1553 cm-1 due to asymmetric stretching in carboxylate anion that is reconfirmed by another peak at 1411 cm-1 which is related to the symmetric stretching mode of the carboxylate anion[20].
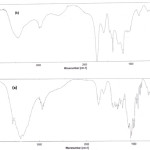 |
Figure 1: FTIR spectra of pure pectin (a) and H-Pec-poly(sodium acrylate) hydrogel (b).
|
Scanning electron microscopy
One of the most important properties that must be considered is hydrogel microstructure morphologies. The surface morphology of the samples was investigated by scanning electron microscopy. Figure 2 shows an SEM micrograph of the polymeric hydrogels obtained from the fracture surface. The hydrogel has a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers.
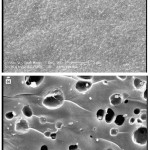 |
Figure 2: SEM photograph of the pure Pectin(a), and H-Pec-poly(sodium acrylate) hydrogel. Surfaces of hydrogel was taken at a magnification of 2000, and the scale bar is 10 μm.
|
Effect of pH on Equilibrium Swelling
equilibrium swelling of H-Pec-poly(sodium acrylate) hydrogel was measured at various buffer solutions with pH ranged from 1 to 13 (Fig. 3). Under acidic pHs, most of the carboxylate anions are protonated, so the main anion-anion repulsive forces are eliminated and consequently swelling values are decreased[19]. At higher pHs (5-9), some of carboxylate groups are ionized and the electrostatic repulsion between COO– groups causes an enhancement of the swelling capacity. Again, a charge screening effect of the counter ions (cations) limit the swelling at higher basic pHs (pHs>9).
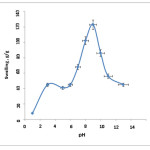 |
Figure 3: Effect of pH of buffered solution on swelling of H-Pec-poly(sodium acrylate) hydrogel. |
pH-responsiveness Behavior of the Hydrogel
In this series of experiments, the pH-dependent swelling reversibility of the H-Pec-poly(sodium acrylate) hydrogel was examined in two acidic and basic buffered solutions. Figure 4 shows the reversible swelling-deswelling behavior of the hydrogel at pHs 3.0 and 9.0. At pH 9.0, the hydrogel swells due to anion-anion repulsive electrostatic forces, while at pH 3.0, it shrinks within a few minutes due to ˝screening effect˝ of excess cations. This sudden and sharp swelling-deswelling behavior at different pH values makes the system to be highly pH-responsive and consequently it may be a suitable candidate for designing controlled drug delivery systems. This behavior has also been observed in the case of commercial acrylic acid-based SAPs(15) as a standard crosslinked polyelectrolyte. Similar swelling-pH dependencies have been reported in the case of other hydrogel systems [21-22].
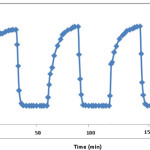 |
Figure 4: On-off switching behavior as reversible pulsatile swelling (pH 9.0) and deswelling (pH 3.0) of the hydrogel. |
Salt-sensitivity of H-Pec-poly(sodium acrylate) hydrogel
The swelling capacity of superabsorbent hydrogels could be significantly affected by various factors of the external solutions such as its valencies and salt concentration. The presence of ions in the swelling medium has a profound effect on the absorbency behavior of the superabsorbent hydrogels. Many theories were reported in the case of swelling behavior of ionic hydrogels in saline solutions. The simplest one of the theories is Donnan equilibrium theory. This theory attributes the electrostatic interactions (ion swelling pressure) to the difference between the osmotic pressure of freely mobile ions in the gel and in the outer solutions. The osmotic pressure is the driving force for swelling of superabsorbents. Increasing the ionic mobile ion concentration difference between the polymer gel and external medium which, in turn, reduces the gel volume, i.e. the gel shrinks and swelling capacity decreases (charge screening effect). In addition, in the case of salt solutions with multivalent cations, “ionic crosslinking” at surface of particles causing an appreciably decrease in swelling capacity. For example, Castel et al. reported that calcium ion can drastically decrease the swelling capacity for a hydrolyzed starch-graft-polyacrylonitrile, due to the complexing ability of the carboxylate group to include the formation of intra- and inter-molecular complexes[23].
In fact,Since the pectin-based hydrogels are comprised poly(NaAA) chains with carboxylate groups that can interact with cations, they exhibit various swelling capacity in different salt solutions with same concentrations. The effect of cation on swelling can be concluded from Figure 5. In salt solution, degree of crosslinking is increased and swelling is consequently decreased. Therefore, the absorbency of the synthesized hydrogel is in the order of H2O > NaCl. In the presence of the bivalent calcium ions, the crosslinking density intensity increases because of a double interaction of Ca2+ with carboxylate groups leading to “ionic crosslinking”. The swelling–deswelling cycle of the hydrogel in sodium and calcium salts are shown in Figure 6. In sodium solution, swelling of the hydrogel is increased with time. When this hydrogel is immersed in calcium chloride solution, it deswells to a collapsed form. When the shrinked hydrogel is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions. This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. As a result, when hydrogel is treated alternatively with NaCl and CaCl2 solutions with equal molarity, the swelling reversibility of hydrogel is observed[21,24].
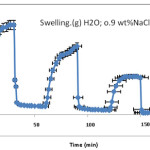 |
Figure 5: swelling–deswelling cycle of the H-Pec-poly(sodium acrylate) hydrogel in distilated water and 0.9 wt% sodium choloride salt.
|
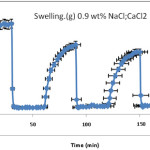 |
Figure 6: swelling–deswelling cycle of the H-Pec-poly(sodium acrylate) hydrogel in sodium choloride and calcium choloride solution(0.9 wt%) . |
Conclusion
A novel superabsorbent hydrogel, H-Pec-poly(sodium acrylate), was synthesized in an aqueous solution by graft copolymerization of acrylic acid onto pectin. The maximum water absorbency in distillated water (348 g/g) was achieved. Swelling measurement in NaCl salt solutions shows a swelling-loss, in comparison with distilled water. This behavior can be attributed to charge screening effect and ionic crosslinking for mono- and multi-valent cations, respectively. In addition, the swelling of hydrogels in solutions with various pHs, exhibited high sensitivity to pH, so that the pH reversibility and on-off switching behavior makes the intelligent hydrogel as a good candidate for considering as potential carriers for bioactive agents, e.g. drugs.
References
- Buchholz, F.L., Graham, A.T. Modern Superabsorbent Polymer Technology, New York: Wiley,(1997).
- Cheng, H., Zhu, J.L., Sun, Y.X., Cheng, S.X., Zhang, X.Z., Zhuo, R.X. Bioconjug. Chem.19:1368-1374(2008).
- Chu, L.Y., Kim, J.W., Shah, R.K., Weitz, D.A. Adv. Funct. Mater.17: 3499-3504(2007).
- Chu, L.Y., Yamaguchi, T., Nakao, S.Adv. Mater. 14: 386-389(2002).
- Crescenzi,V.,Cornelio,L.,DiMeo,C.,Nardecchia,S.,Lamanna,R. Biomacromolecules.8, 1844-1850(2007).
- J. Wu, J. Lin, M. Zhou, C. Wei, Macromol. Rapid Commun.21, 1032-1034(2000).
- Eddington, D.T., Beebe, D.J.Adv. Drug Deliv. Rev. 56: 199-210(2004).
- J. Lin, J. Wu, Z. Yang, M. Pu, Polymers & Polymer Composites.,9, 469-471(2001).
- J. Wu, Y. Wei, J. Lin, S. Lin, Polymer. 44, 6513-6520(2003).
- Hoffman, A. S. Polymeric Materials Encyclopedia;, Salamone, J. C.; Ed.; CRC Press, Boca Raton, FL.5, 3282(1996).
- Kirk RE, Othmer DF. Encyclopedia of Chemical Technology, Vol. 4, Kroschwitz JI, Howe-Grant M. (eds). John Wiley & Sons: New York, 942. (1992).
- Barvic, M.; Kliment, K.; Zavadil, M. J. Biomed Mater Res.1, 313–323(1967).
- Chirila, T. V.; Constable, I. J.; Crawford, G. J.; Vijayasekaran, S.; Thompson, D. E.; Chen, Y. C.; Fletcher, W. A.; Griffin, B. J. Biomaterials.14, 26–38(1993).
- G.F. Fanta, W.M. Doane, Grafted Starches, in: Modified Starches: Properties and Uses, Q.B. Wurzburg, (Ed.), CRC Press, Boca Raton (Florida), p. 149(1986).
- Yamaguchi, M.; Watamoto, H.; Sakamoto, M. Carbohydr Polym 9, 15(1988).
- Pourjavadi A, Harzandi AM, Hosseinzadeh H.Eur. Polym. J.40:1363(2004).
- Flory PJ. Principles of Polymer Chemistry, Ithaca, Cornell University Press, New York,(1953).
- Lim, D.W.; Whang, H.S.; Yoon, K.J. J Appl Polym Sci, 79, 1423–1430(2001).
- Hosseinzadeh, H.; Pourjavadi, A.; Zohouriaan-Mehr, M. J.; Mahdavinia, G. R. J Bioact Compat Polym, 20, 475-491(2005).
- Silverstein RM, Webster FX. Spectrometric Identification of Organic Compounds, 6th Edn., Wiley, New York,(1998).
- G. R. Mahdavinia, A. Pourjavadi, H. Hosseinzadeh, M.J. Zohuriaan-Mehr, Eur. Polym. J. 40, 1399-1407(2004).
- Chen J, Zhao Y.J Appl Polym Sci.75:808(2000).
- Lim DW, Whang HS, Yoon KJ. J. Appl. Polym. Sci. 79:1423(2001).
- Barbucci R, Maganani A, Consumi M.Macromolecules; 33:7475(2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.









