Synthesis and Characterisation of Bidentate Schiff Base Derived From Furfuraldehyde and its Transition Metal Complexes
Archana Saxena
Department of Chemistry, M.I.T.Moradabad (India)
The schiff base ligand was prepared from furfuraldehyde with 4-amino-5-mercapto-5-triazole and characterised. The metal complexes of Ti(III),V(III),Mn(III),Co(III) and Ru(III) derived from this ligand were characterised by elemental analyses, molar conductance,magnetic measurements, infra red 1H NMR & electronic spectral data. Based on these studies octahedral structures have been assigned to these complexes.
KEYWORDS:Furfuraldehyde; Triazole and Octahedral
Download this article as:| Copy the following to cite this article: Saxena A. Synthesis and Characterisation of Bidentate Schiff Base Derived From Furfuraldehyde and its Transition Metal Complexes. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Khan M. A, Ahmad S. Microwave Assisted Synthesis of Indole Derivatives, an their Complexation Behaviour and Biological Studies. Available from: http://www.orientjchem.org/?p=23547 |
Introduction
Schiff bases are an important class of ligands in coordination chemistry and their complexing ability containing different donor atoms is widely reported.1-4 The ligands resulting from 4-amino-3-ethyl-5-mercapto-5-triazole(AMT) with benzaldehyde and 2-hydroxy-1-naphthaldehyde have been reported.5,6 A servery of chemical literature reveals that very little work appears to have been done on the synthesis and characterisation of the schiff bases derived from furfuraldehyde and AMT and there fore it was thought to be of intrest to extend the studies of this schiff base to the synthesis of complexes with Ti(III),V(III),Mn(III),Co(III)& Ru(III)
Experimental
All the chemicals used were of A.R.grade or equivalent purity. Ethanol and ether were distilled before use.Furfuraldihyde (sisco) and metal chlorides were procured from reputed firms and used as such except Ti(III)chloride which was prepared in the lab by reported method.
Preparation of the Ligand
4-amino-3-ethyl-5-triazole was prepared by reported method.6 The schiff base was prepared by refluxing the equimolar mixture of furfuraldehyde and triazole in ethanol for about 3h. The reaction mixture was then kept over night at room temperature, when the crystals of the schiff base were obtained. These were filltered and washed with ethanol. The product was purefied by recrystallisation from ethanol. The purity of the ligand was checked by TLC.
Preparation of the Complexes
The solution of the metal salt was added dropwise to the hot ethanolic solution of the ligand in the molar ratio of 1:2 with constant stirring. Immediate precipitation resulted in each case. The precipitate was filtered, washed and dried in vacuum desicator.
Characterisation of Ligand and Metal Complexes
The melting points of ligand and its metal complexes were determined by open capilary method and are uncorrected. C,H& N were estimated by microanalysis. Metals were estimated gravimetrically in the lab. The IR spectra were recorded on a Beckmann IR-20 spectrophometer in the range of 4000-250cm-1
The electronic spectra of the complexes were recorded by Beckmann- DU spectrometer. The magnetic susceptibility was determined by Gouy’s method using CuSO4.5H2O as calibrant.
Results and Discussion
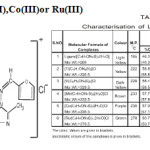
The analytical data for the complexes suggested 1:2 stiochiometry for all the synthesised complexes. The vast difference in the melting point of the ligand and its corresponding metal complexes indicated the formation of the complexes. The complexes were stable in air and non-hygroscopic. These are insoluble in common organic solvents but soluble in nitrobenzene, acetonitrite, DMF & DMSO.
The determination of molar conductance at 25oC and 10-3M dilution suggested 1:1 electrolytic nature for all the complexes.
The IR spectrum of the ligand shows characteristic NH and S-H bands at 3160and 2600cm-1 respectively.8 Another band at 1090 cm-1 is assigned to (C=S). The deprotonation of thiol group and complexation through sulphur is indicated by the absence of band at 2600cm-1 due to SH in the spectra of complexes. Metal-sulphur bond9,10 formation is further confirmed by a band at 380-340cm-1 in the far IR region, A new band appears in the region of 760cm-1 which may be assigned to (C-S). This further supported the coordination through sulphur atom. A strong band in the region 1620cm-1 in the free ligand assigned to (-N=CH is lowered by 20-30cm-1 in the spectra of the complexes, suggesting coordination through azomethine nitrogen atom of the schiff base(II). This is further supported by metal-nitrogen band in the region of 540-480cm-1.12.
The presence of coordinated water molecules is suggested by a broad band in the region of 3400-3450cm-1 and supported by wagging and rocking modes in the region of 840-850 and 740-755cm-1 respectively.13 It is also supported by TGA.
The 1H NMR spectrum of the ligand shows signal due to SH protons at 9.70 which disappears in the speetra of the corresponding M(III) complexes. The signal at 7.35 due to azomethine proton has shifted in the spectra of the complexes. These changes in the 1H NMR spectra indicated chelation of the ligand through sulphur and azomethine nitrogen.
The electronic spectrum of Ti(III) complex shows a single broad band at 19560 cm-1 due to 2T2g2Eg transitions for Oh symmetry.14 The value of magnetic moment of this complex is 1.68B.M. which is in the expected range for d1 system like Ti(III) and shows paramagnetic character of the complex. It also shows that Ti(III) has not been oxidised to Ti(IV) during or after complexation, although its is very sensitive to oxidation.
The Mn(III) complex shows a magnetic moment of 4.93B.M. corresponding to the presence of four unpaired electrons and high spin state of the complex. This value also suggests the absence of any kind of exchange interaction.15 the electronic spectrum of complex shows an intense and sharp charge transfer band at 22000cm-1 and a spin allowed d-d transition at 18540cm-1,characterstic of octahedral geometry.15
The study of magnetic properties of the Co(III)complex indicated diamagnetic nature, as expected for a low spin d6 ion. The electronic spectrum of the chelate displays bands at 15230, 21195 and 23410cm-1,assignable to 1A1g3T2g, 1A1g1T1g and 1A1g1T2g transitions respectively. These are characteristic of low spin octahedral complexes of Co(III).16
The observed value of effective magnetic moment of the complex is 2.92B.M. as expected for d2 system like V(III). The electronic spectrum the complex exhibits a band at 16110cm-1 with a shoulder at 20530cm-1. The low energy band may be assigned to 2A1g3T2g and the high energy band to 2A1g3T2g(p) transition. These are characteristic of octahedral geometry.17
The Ru(III) complex shows a magnetic moment of 1.98 B.M. and three bands in its electronic spectrum at 13650,17600 and 22500 cm-1.These band are assigned to 4T2g4T1g, 4T2g4T2g & 2T2g2A2g,2T1g respectively. These are similar to those reported for other Ru(III) octahedral complexes.18
Conclusion
Based on elemental analyses, molar conductance magnetic and spectral data,octahedral geometry has been proposed for all the synthesised complexes.
 |
Scheme 1 Click here to View scheme |
References
- Bhattacharya P.J.,Indian Chem. Soc., 59, 505(1982).
- Samy CR,Indian J.Chem,35A,(1996).
- Shen X,Yang Q.I.C.& XieY,Synth. react inorg met org chem,26,1135(1996).
- Singh K, Dubey S N& Tandon J P,Synth. react inorg met org chem,23,1251(1993).
- Gadag R V, Gajendragad M R, Curr Sci,48,839(1979).
- Dhaka K S, Mohan J, Chadha V K & Pujari H.K., Indian J Chem, 12,288(1974).
- Vogel A I, A text book of quantitative inorganic analysis, 4th Edn (Longmanns Green, London).
- Ashok K Sen, Gurmit Singh, Kiran Singh, Raj K.Naran, ram N Handa & Surendra N Dubey, Indian J Chem, 36A, 891(1997).
- Karuna Mahajan, Nighat Fahmi & Ran Uir Singh, Indian J Chemistry 46A,1221(2007).
- Gajandragad M R & Agrawal U, Aust. J Chem,28,763(1975).
- Suzuki I, Bull Chem Soc Japan,35,1286 (1962).
- Kamal M Ibrahim, Sahar I Mostafa, Nagwar & Zeinab A Younis, Indian J chemistry,43A, 2294 (1904).
- A. Kirza, A. Reiss, S. Florea & T.Caproice,J.Indian Chem.Soc,77,207(2007).
- Samik K Gupta, S. Roy, T. N. Mandal, K.Das & S. Ray J. chem Sci,122,239(2010).
- Rahul K Rastogi, Poonam Garg & Shamim Ahmad, Asian J Chem., 21,6144(2009).
- T. Daniel Thangaduvai & K. Natrajan, Indian J.Chem., 41A, 741(2002).
- Kamalendu Dey, P.K. Bhattacharya, D.Bandyopadhyay & K.Chakraborty, Indian JChem., 35A, 580(1996).
- Mahesh K Singh, A. K. Singh, P.K. Gupta Jaipal & L.K. Sharma, Indian J. Chem., 41,1385(2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









