Study of Some Biorelevant Complexes of Transition Metals with Active Imines
M. Haseen, Pramila Sharma and S. K. Sharma
Department of Chemistry, Bareilly College, Bareilly - 243 005 (India).
The reactions of transition metal salts with monofunctional bidentate thiosemicarbazone (TSCZH) derived from the condensation of 6-nitro-1H-indole 2-3 dione with thiosemicarbazide have been carried out in 1:2 molar ratio. Derivatives of the type [M(TSCZH)2 .xH2O] Cl (where M=Ti (III), V (III), Mn (III), Co (III), Ru (III), Fe (III) Mo O2(V) and Mo O2(VI) have been obtained. All these complexes are coloured solids and monomeric in nature. Octahedral geometry has been proposed for these complexes based on elemental analyses, molar conductance, magnetic susceptibility and spectral studies. Antimicrobial effects of the ligand and its complexes on different species of pathogenic fungi and bacteria have been recorded.
KEYWORDS:Transition metal; Octahedral fungi; antimicrobial and biorelevant
Download this article as:| Copy the following to cite this article: Haseen M, Sharma P, Sharma S. K. Study of Some Biorelevant Complexes of Transition Metals with Active Imines. Orient J Chem 2012;28(2). |
| Copy the following to cite this URL: Haseen M, Sharma P, Sharma S. K. Study of Some Biorelevant Complexes of Transition Metals with Active Imines. Available from: http://www.orientjchem.org/?p=23520 |
Introduction
There is substantial interest in the therapeutic activities of the co-ordination compounds as drugs1-3. In recent years some important Schiff base complexes derived from 2,3-diaminopyridine and O-vanillin were synthesized and their antibacterial activity has also been studied4. Schiff bases act as intermediate in biological processes. Examples of such processes are reactions involving pyridoxal enzyme, aldol condensation, decarboxylation, transmutation and visual processes.
A lot of work is reported on the Schiff base hydrolysis, but the mechanism of the hydrolysis has not been worked out in detail5-6. Several Schiff base complexes have been synthesized as models for some of metalloproteins7-9. Schiff base complexes have gained additional importance due to their possible applications as superoxide dismutase mimics10-11 and their catalytic role in the expoxidation of olefins12.
Transition metals form some specific and important drugs with diphenyl pyridine and isothipendyl. These drugs are found to be antiserothenine, antihistaminic, anticonvulsants and antifungal in nature and hence used in medicinal chemistry13-15. It has been well established that certain platinum and palladium16-17 complexes are of biological importance due to their carcinostatic activity and interest in biological chemistry. Chalson et al 18 have reported that cesium cis-dichlorosreinetopalladate (II) induces filament growth in E. Coli. The complexes of palladium (II) with amino acids such as glycine, serine and glutamine have also been reported 19-20 to be active against certain tumors. It is also well known that carcinostatic action21-24 of drugs is due to their intereaction with nuclear D.N.A. Keeping these facts in view, the present research work was carried out.
Experimental
The transition metal salts and m-nitroaniline were purchased from Lobochemie and used as such. 6-Nitroisatin was prepared in the laboratory. All the solvents were dried and distilled before use.
6-Nitroisatin was synthesised by Sandmeyer isonitrosoacetanilide synthesis method.25. The ligands TSCZH was prepared by the condensation of 6-nitroisatin with hydrazinecarbothioamide in presence of sodium acetate in 1:1 molar ratio, in absolute alcohol. The reaction mixture was refluxed over a water bath for 4h and allowed to stand overnight. The product was recrystallized from ethanol and dried in vacuo. It may be represented by following structure.
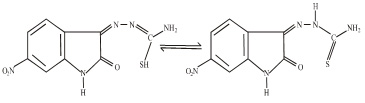
Synthesis of Metal Complexes:
The ethanolic solution of metal salts and ligand was mixed in 1:2 molar ratio. Aqueous NH4OH was added dropwise in the reaction mixture till the solution became basic (pH 8.0). The mixture was then stirred on a magnetic stirr for 2-3 h and the resulting product was recovered by filteration, washed with ethanol and dried in vacuo.
The ligand and its metal complexes were subjected to elemental analyses for C,H and N, while metal was estimated gravimetrically in the lab (Sulphur was estimated by Messengers method). The melting points were determined by open capilary method and are uncorrected. The molar conductance was determined by Systronics (model 305) conductivity bridge. Magnetic sysceptibity was determind by Gouy’s method, using CuSO4.5H2O as calibrant. The electronic spectra were recorded by Backmen -DU Spectrophotometer. The IR spectra were recorded in KBr pellets at CDRI Lucknow.
Antimicrobial Activity
The antifungal activity was evaluated against Macrophomina phaseolina and Fussarium oxysporum using standard food poisoning technique and a procedure recommended for testing new chemicals26. The Linear growth of the fungus was recorded by measuring the diameter of the fungus colony after 96h and the percentage inhibition was calculated as 102 (dC -dT)/dC. Where dC and dT are the diameters of the fungus colony in the control and test plates, respectively.
Antibacterial activity was tested against Bacillus substiles and Salmonella species using the paper disc plate method.27
Results and Discussion
The analytical data suggested 1:2 (M:L) stochiometry for the synthesized complexes. The vast difference in melting point of the ligand and its complexes indicated their formation. The determination of molar conductance suggested 1:1 electrolytic nature for all the M(III)& MoO(V) complexes except those of Ru (II) & MoO2(VI) which are non electrolytes.
The I.R. spectrum of free ligand shows bands at 3284,1612 and 995 cm-1 which may be assigned to n (N-H), n (C=N) and n (C=S) respectively. The band due to N-H vibration disappears in IR spectrum of the complexes indicating deprotonation of the functional group upon complexation28. The band at 1612 cm-1 shifted to higher wave number by 10-15 cm-1 in the metal complexes, which suggested coordination through azomethine nitrogen atom. The band at 995 cm-1 is shifted towards lower frequencies indicating involvent of C=S group in co-ordination. The band at 3430 cm-1 attributed to asymmetric and symmetric mode of NH2 group remain almost at the same position in the spectra of the complexes, suggesting non-involvent of these groups in bonding29. In the far IR region of the complexes, some non ligand bands have appeared in the region of 440-445 & 365-375 which may be assigned to n (M-N) and n (M-S) respectively. The appearance of these bands further support the bonding of the ligand to the metal atom through nitrogen and sulphur atom. Thus the ligand is behaving in mono basic bidentate manner. In the case of Mo O(V) complex a band has appeared at 950 cm-1 due to Mo =O stretching frequency. The strong band exhibited by the dioxomolybdenum (VI) complexes in the region 950-970 and 910 Cm-1 are attributed to Vsym (O = Mo = O) and Vsym (O = Mo = O) respectively of Cis Mo O2 configuration due to the maximum utilization of the available p orbitrals for bonding with oxogroups. The I.R. spectra of the complexes show non-ligand bands in the region of 3480-3510 cm-1 due to n O-H and 840-850 cm-1 assignable to rocking mode of coordinated water molecules. TGA also supported the coordinated nature of water molecules.
The H1 NMR spectrum of the ligand shows, multiplets at 6.70 ppm due to aromatic protons which remain in same position in the metal complexes. The free ligand exhibit sharp singlet at 11.25 ppm due to -NH proton, which disappear in the metal complexes, suggesting deprotonation during complexation.
Electronic Spectra
The electronic spectrum of Ti (III) complex shows a single broad band at 19560 cm-1 due to transitions for Oh symmetry30. The value of magnetic moment of this complex is 1.68 B.M. which is in the expected range for d1 system like Ti(III) and shows paramagnetic character of the complex. It also shows that Ti (III) has not been oxidised to Ti (IV) during or after complexation, although it is very sensitive to oxidation.
The Mn (III) complex shows a magnetic moment of 4.93 B.M., corresponding to the presence of four unpaired electrons and high spin state of the complex. This value also suggests the absence of any kind of exchange interaction31. The electronic spectrum of complex shows an intense and sharp charge transfer band at 22000 cm-1 and a spin allowed d-d transition at 18540 cm-1 characterstic of octahedral geometry31.
The study of magnetic properties of the Co (III) complex indicated diamagnetic nature, as expected for a low spin d6 ion. The electronic spectrum of the chelate displays bands at 15230. 21195 and 23410 cm-1 assignable to , and transitions respectively. These are characteristic of low spin octahedral complexes of Co (III)32.
The observed value of effective magnetic moment of the complex is 2.92 B.M., as expected for d2 system like V (III). The electronic spectrum of the complex exhibits a band at 16110 cm-1 with a shoulder at 20530 cm-1. The low energy band may be assigned to and the high energy band to . These are characterized of octahedral geometry33.
The Ru (III) complex shows a magnetic moment of 1.98 B.M. and three bands in its electronic spectrum at 13650. 17600 and 22500 cm-1. These bands are assigned to . and , respectively, and are similar to those reported for other Ru (III) octahedral complexes34.
The electronic spectrum of oxomolybdenum (V) complex suggested that the complex may be considered as octahedral with a strong tetragonal distortion resulting from Mo=O bond. The spectrum exhibited three distinct absorption bands in the ligand field region. The low intensity band at 13000 cm-1 in the long wavelength region is possibly due to first crystal field transitin (dxy, dyz, dxz). The second crystal field transition at 19000 Cm-1 is assignable to (dxy, dx2 – y2). The third peak was observed at 3000 assignable to (dxy, d z2 ).
The electronic spectrum of Mo O2 (VI) complex has a single band due to charge transfer transition. Its complex is diamagnetic in nature as axpected for do configuration.
The Ru (II) complex is diamagnetic in nature which shows +2 oxidation state for Ru (II) in this complex. The electronic spectrum of the complex in CH2Cl2 shows a band assigned to the charge transfer transition arising from the excitation of an electron from the metal t2g level to the unfilled molecular orbitals derived from the p* level of the ligand in accordance with the assignments made for other similar octahedral Ru (II) complex35.
The magnetic moment of Fe (III) complex is 5.87 B.M. corresponding to the presence of five unpaired electrons and high spin state of Fe (III) in this complex. The electronic spectrum of this complex shows three bands at 12720, 19600 and 25000 cm-1 due to (G), (G) and (G) transition respectively in an octahedral symmetry.36
Conclusion
On the basis of studies performed and discussed above octahedral geometry have been proposed for all the synthesized complexes.
 |
Scheme 1 Click here to View scheme |
Antifungal & Antibacterial Activities
The data for the antifungal and antibacterial activities of the ligands and their corresponding compounds evaluated against Fusarium oxysporum and Alternaria alternata and bacteria Escherichia coli and Pseudomonas cepacicola have been recorded. The results point out that the compounds are inhibiting the growth of fungus and bacteria to a greater extent as the concentration is increased. The enhanced activity of metal chelates may be ascribed due to the increased lipophilic nature of these complexes arising due to chelation37. The toxicity increases as the concentration is increased. Mode of action of antimicrobials may involve various targets in microorganisms, e.g. interference with the cell wall synthesis, damage to the cytoplasmic membrane as a result of which cell permeability may be altered or they may disorganize the lipoprotein leading to the cell death38.
Table 1 : Analytical & Physical Data of Ligand & Complexes
|
Sl No |
Compound
|
Colour |
M.P. 0C |
Elemental analyses |
Magnetic Moments in (B.M.) |
Molar Conductance ohm-1 cm2 mole-1 |
||||
|
% of C |
% of H |
% of N |
% of S |
% of M |
||||||
| 1 | (TSCZH) |
Grey |
221 |
40.90 (40.85) |
2.27 (2.25) |
26.51 (26.49) |
12.12 (12.09) |
– |
– |
– |
| 2 | [Ti (C9H6N5O3S)2 .2H2O] Cl |
Yellow |
275 |
33.35 (33.31) |
2.47 (2.41) |
21.62 (21.59) |
9.88 (9.82) |
7.41 (7.39) |
1.68 |
65 |
| 3 | [V(C9H6N5O3S)2 .2H2O] Cl |
Yellow |
278 |
33.20 (33.18) |
2.46 (2.44) |
21.52 (21.45) |
9.84 (9.80) |
7.84 (7.81) |
2.92 |
70 |
| 4 | [Mn (C9H6N5O3S)2 .2H2O] Cl |
Brown |
280 |
33.00 (32.96) |
2.43 (2.42) |
21.39 (21.35) |
9.77 (9.71) |
8.40 (8.37) |
4.93 |
75 |
| 5 | [Fe (C9H6N5O3S)2 .2H2O]Cl |
Dark Brown |
276 |
32.95 (32.90) |
2.44 (2.41) |
21.35 (21.31) |
9.76 (9.74) |
8.54 (8.52) |
5.87 |
65 |
| 6 | [Co (C9H6N5O3S)2 .2H2O]Cl |
Brown |
281 |
32.80 (32.78) |
2.42 (2.40) |
21.46 (21.23) |
9.71 (9.69) |
(8.95 (8.91) |
Diamagnetic |
70 |
| 7 | [MoO (C9H6N5O3S)2 .H2O]Cl |
Redish Brown |
295 |
30.36 (30.33) |
1.96 (1.94) |
19.67 (19.60) |
8.99 (8.95) |
13.48 (13.45) |
1.78 |
75 |
| 8 | [MoO2(C9H6N5O3S)2 ] |
White |
286 |
32.92 (32.87) |
1.82 (1.79) |
21.34 (21.30) |
9.75 (9.71) |
14.62 (14.58) |
Diamagnetic |
Non Electrolyte |
| 9 | [Ru(C9H6N5O3S)2 .2H2O]Cl |
Green |
310 |
30.83 (30.80) |
2.28 (2.25) |
19.98 (19.95) |
9.14 (9.12) |
14.42 (14.39) |
1.98 |
80 |
| 10 | [Ru(C9H6N5O3S)2 .2H2O] |
Green |
305 |
42.48 (42.45) |
2.40 (2.38) |
21.05 (21.00) |
9.62 (9.60) |
15.18 (15.12) |
Diomagentic |
Non electrolyte |
References
- Reedijik J., Pure Appl Chem. 59 (1987) 181.
- Lohrer PJ & Einhorn LH, Am Intern Med 100 (1984) 704.
- Bhave N S, Bahad PJ, Sonparote PM and AswarAS, Journal Indian Chem. Soc. April (2002) 79, p342.
- Henri Likam Wah, Tagenine J & Cupta BM Indian J Chem. 40(A) (2001) 999.
- Senapati S, Dash PK, Mishra BK, Behra GB, Indian J. Chem. 34A (1995) 278.
- Sudalaindi Kumaresan, palani Ramdei and Shikh Mobin, Indian Jounal Chem. 43(A) 2004 1664-1667.
- Kipke CA, Scott MJ, Gbdes JW and Arm strong AW, Inorg chem 29 (1999) 2197.
- Dialey GC, Horwitz CP & LisekCA, Inorg. Chem. 31 (1992) 5325.
- Ashmawy FM, Me Au Little CA, Parish RV & Tames J, J. Chem Soci Chem Commun 14 (1984) 735.
- Baudry M, Etiena S, Brace A, Palucki M, Jacobson E & Malfary B, Biochem Biophys Res Commun 192 (1993) 964.
- Prasad RM, Mithilesh, Agrawal and Sanjana Sharma, Indian J. Chem. 43 A (2004) 337-340.
- Agrawal DD., Jain D, Rastogi R & Agrawal V, J Indian Chem. Soc. 73 (1996) 580.
- Ramappa PG & Somase kharappa KB, J Indian Chem. Soc. 33(A) (1994) 66..
- Biradar MS, Patil CS & Rudzinski WL, Inorg. Chem Acta 78 (1987) 107.
- Wen Tao Gao & Zhuo Zhang, Pure Appl. Chem. 77, (2002) 1559-1574.
- Rosenberg B, Vancamp L, Trosko JE & Mansour VH, Nature Landon 222 (1969) 385.
- Biyala MK, Fahmi N, & Singh RV, Indian J. Chem. 43(A) (2004) 2536.
- Charlson AJ, Banner RJ, Gale RP, Me ArdleNT, Trainor KE & Walton E C, J Clin Hematol Oncol 7 (1977) 294.
- Charlson AJ, ArdleNJ & Walton EC, Inorg Chem. Acta 56 (1981) 35.
- Trainor KE & Walton EC, Cancer Treat Rep. 61 (1977) 469.
- Rosenberg B., Biochenie 60 (1978) 859.
- Hankare PP, Narvane SR, Bhuse VM, DelekarSD & Jagtap AH, Indian J Chem. 43(A) 2004, 1660-1669.
- Garoufis A, Kasselouri S, Gerlepes SP, Indian J. Chem. 46(A) (2006) 1510-1560.
- Banerjee J, Boxe D, Hafizur Rehman, Bailey SK, Rosa D., Walash Ind. J. Chem. 43(A) 2004 1119-1122.
- Garg R, Saini MK, Fahmi N &Singh RV, Indian J. Chem. 44(A) (2005) 2433.
- Methods for evaluting Plant Fungicides and Bactericides (AM Phytopathol Soc. Minnisota, USA, (1978) 141.
- Fahmi N & Singh RV, Trans. Met Chem. 19 (1994) 12.
- Biyala MK, Fahmi N and Singh RV, Ind. J. Chem. 45(A) 2006 (1999-2005).
- Biyala MK, Fahmi N & Singh RV, Indian J. Chem 43(A) (2004) 1662.
- Samik K Gupta, Roy S, Mandal TN, Das K., J. Chem. Sci. 122 (2010) 239.
- Rahul K Rastogi, Poonam Garg & Shamim Ahmad, Asian J. Chem. 21 (2009) 6144.
- T. Daniel Thangaduvai & K. Natrajan, Indian J. Chem. 41(A) (2002) 741.
- Kamalendu Dey, Bhattacharya PK, Bandyo Padhyay & Chakraborty K, Indian J. Chem. 35(A) (1996) 580.
- Mahesh K. Singh, Singh AK, Gupta PK, Jaipal & Sharma LK, Indian J. Chem. 41 (2002) 1385.
- Natrajan K, Poddar RK and Agrawal U, J Inorg. Nucl. Chem. 39 (1977) 431.
- Liver AB, Inorganic electronic spectroscopy and Adn. (Exevier, Amsterdam) 1984 and references therein.
- Gandhi N, Jain R & Kaushic NK, Thermo Chem. Sci 2 (2) (2004) 147.
- Dwivedi P. Singh V, Fahmi N & Singh RV

This work is licensed under a Creative Commons Attribution 4.0 International License.









